What is Sodium silicate?
Sodium silicate is an inorganic sodium salt which has silicate as the counterion. It is also called Sodium metasilicate or Waterglass. The chemical formula of Sodium silicate is (Na2O)x·SiO2.
Sodium metasilicate is a flaked solid or powdered substance. It dissolves in water to produce alkaline solutions. It has a polymeric anion. In alkaline and neutral solutions it is stable whereas, in acidic solutions, the silicate ions will react with hydrogen ions and form silicic acids, which are likely to decompose into hydrated silicon on the dioxide gel. When further heated, it drives off the water and a hard translucent substance called silica gel is obtained.
Table of Contents
- Properties of Sodium silicate – (Na2O)x·SiO2
- Structure of Sodium silicate –(Na2O)x·SiO2
- Uses of Sodium silicate –(Na2O)x·SiO2
- Production of Sodium silicate
- Health Hazards
- Frequently Asked Questions
Properties of Sodium silicate – (Na2O)x·SiO2
| Sodium silicate | (Na2O)x·SiO2 |
| Molecular weight of Sodium silicate | 122.062 g/mol |
| Number of hydrogen bond acceptor | 3 |
| Complexity | 18.8 |
| Number of covalent bonds | 3 |
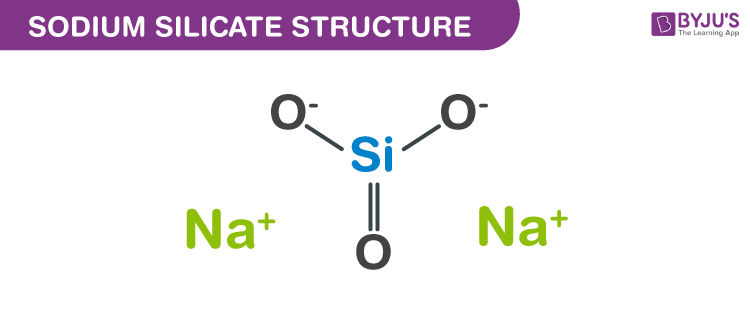
Structure of Sodium silicate ((Na2O)x·SiO2)
Uses of Sodium silicate ((Na2O)x·SiO2)
- Sodium silicate is used in wastewater treatment plants as an iron flocculant and an alum coagulant.
- It is used as hand-dyeing as a fixative.
- It is used in Pottery.
- It is widely used in food preservation, homebrewing, and aquaculture.
Production of Sodium silicate
Sodium silicate solution can be prepared in a reactor by treating a mixture of silica, water, and caustic soda with hot steam. The reaction is as follows:
2x NaOH + SiO2 → (Na2O)x·SiO2 + x H2O
Sodium metasilicate can also be produced by dissolving silica SiO2 in molten sodium carbonate (Na2CO3) at a temperature of 851 °C
x Na2CO3 + SiO2 → (Na2O)x·SiO2 + CO2
Health hazards
It is a powerful irritant to eyes, mucous membrane, and skin. When this inorganic salt is ingested it can be toxic. Inhaling this compound causes sore throat, coughing, burning sensation, and shortness of breath.
Frequently Asked Questions – FAQs
What are the uses of sodium silicate?
How can sodium silicate be prepared?
Why is sodium silicate employed in the treatment of concrete?
Learn more about the physical and chemical properties of Sodium silicate ((Na2O)x·SiO2) from the experts at BYJU’S.ts at BYJU’S.

Comments