What is Sodium Thiosulfate?
Sodium thiosulfate, which is also referred to as sodium sulphate, is a chemical compound that has the formula Na2S2O3.
It is typically found in its pentahydrate form which is either white in colour, or colourless altogether. This pentahydrate of sodium thiosulfate is described by the following chemical formula: Na2S2O3.5H2O.
Table of Contents
- Sodium Thiosulfate Structure
- Properties
- Important Questions on Sodium Thiosulphate
- Uses
- Hypo Solution Formula
- Production of Sodium Thiosulfate
- Frequently Asked Questions – FAQs
Sodium Thiosulfate Structure
In its solid form, it is a crystalline solid which has a tendency to readily lose water. Sodium thiosulfate is readily soluble in water and is also referred to as sodium hyposulfite. The structure of the Na2S2O3 molecule is illustrated below.
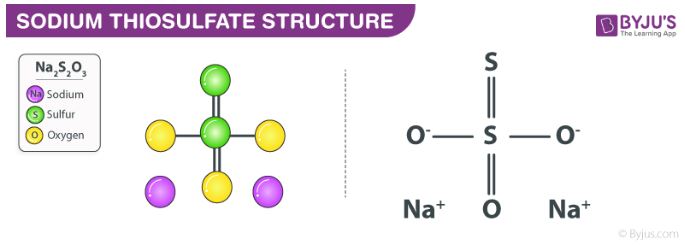
Sodium Thiosulfate Structure
It can be noted that the shape of the thiosulfate ion is tetrahedral in the solid-state of sodium thiosulfate. The distance between the two sulphur atoms in the thiosulfate ion is comparable to the distance between two sigma bonded sulphur atoms. This implies that the sulphur which is not bonded to any oxygens holds a negative charge.
Properties of Sodium Thiosulfate
The physical and chemical properties of Na2S2O3 are listed below.
1. Physical Properties
- In its anhydrous form, sodium thiosulfate has a molar mass of 158.11 grams per mole. The more commonly available pentahydrate from, Na2S2O3.5H2O has a molar mass of 248.18 g/mol.
- It has a white, crystalline appearance as a solid and is odourless.
- The density of sodium thiosulfate corresponds to 1.667 grams per cubic centimetres.
- The pentahydrate of this salt has a melting point of 321.4 K and a boiling point of 373 K.
- The solubility of sodium thiosulfate in water is 70.1g/100 mL at 20oC and 231g/100mL at 100o
- The crystal structure of Na2S2O3 crystals is monoclinic.
2. Chemical Properties
- The sodium thiosulfate salt is neutral in charge. However, it dissociates in water and some other polar solvents to yield Na+ and S2O32-
- Despite being stable at standard conditions, the sodium thiosulfate salt decomposes at high temperatures to yield sodium sulfate along with sodium polysulfide.
- The chemical equation for the reaction described above is given by
4Na2S2O3 ⟶ 3Na2SO4 + Na2S5
- When exposed to dilute acids such as dilute hydrochloric acid, the sodium thiosulfate salt undergoes a decomposition reaction to yield sulphur along with sulphur dioxide.
Na2S2O3 + 2HCl ⟶ 2NaCl + SO2 + H2O + S
- The alkylation of Na2S2O3 yields S-alkyl thiosulfates. These compounds are commonly referred to as Bunte salts.
Important Questions on Sodium Thiosulphate
Comment on the solubility of sodium thiosulphate
Sodium thiosulphate is a colourless monoclinic crystal or a crystalline white powder which is odourless and salty. The relative density for this is 1.667. Water-soluble, Its solubility at 100° C is 231 g/100 ml of vapour. Insoluble in spirits.
Is sodium thiosulphate harmful?
Acute potential health effects of sodium thiosulphate are listed below.
- Skin: This compound may cause mild skin irritation.
- Eyes: This compound can cause mechanical eye irritation.
- Inhalation: sodium thiosulphate can cause upper respiratory tract and mucous membrane irritation.
- Ingestion: this compound is an agent with a low order of toxicity.
How does sodium thiosulphate react with hydrochloric acid?
When reacted with dilute hydrochloric acid, sodium thiosulphate gives sulphur dioxide, water, and sulphur as the product.
Production of Sodium Thiosulfate
- The laboratory preparation of the sodium thiosulfate salt involves the heating of aqueous sodium sulfite solutions along with sulphur.
- The production of Na2S2O3 can also be accomplished by the boiling of aqueous NaOH (sodium hydroxide) with sulphur.
- The reaction for the method described above is given by
6NaOH + 4S ⟶ Na2S2O3 + 2Na2S + H2O.
- Industrially, sodium thiosulfate is prepared from the liquid waste generated from the manufacture of sulphur dye.
It can be noted that upon heating with Al3+ containing samples, sodium thiosulfate gives a white-colored precipitate. This is due to the reaction between the aluminium cation and the thiosulfate anion which forms aluminium hydroxide along with sulphur and sulphur dioxide.
Uses of Sodium Thiosulfate
Some important uses of sodium thiosulfate are listed below.
- Na2S2O3 is a very important chemical compound in the medical treatment of cyanide poisoning cases.
- Sodium thiosulfate is also used medically to treat dermatophytosis (ringworm) and tinea versicolor.
- The side effects of chemotherapy and hemodialysis (purification of blood) are treated with Na2S2O3.
- It is a very important compound in analytical chemistry since it stoichiometrically reacts with iodine to reduce it to the iodide ion while it is oxidized to the S4O62- (tetrathionate) ion.
- Sodium thiosulfate salts are used as photographic fixers due to the ability of the thiosulfate ions to react with silver halides, which make up photographic emulsions.
- Ammonium thiosulfate and sodium thiosulfate make up lixiviants (liquid mediums used in hydrometallurgy) which are used in the extraction of gold from its ores.
- In order to reduce the chlorine levels in water bodies, Na2S2O3 is used in the dechlorination process.
Medical uses of Sodium Thiosulfate
- Sodium thiosulfate, commonly known as sodium thiosulphate, is a medicine that is used to treat cyanide poisoning, pityriasis versicolor, and cisplatin side effects. It is frequently used after the drug sodium nitrite for cyanide poisoning and is usually only prescribed in severe situations.
- Sodium Thiosulfate is a toxin with a modest toxicity level. Large dosages may produce nausea, vomiting, abdominal cramps, diarrhoea, metabolic acidosis, and hypernatremia, as well as gastrointestinal discomfort.
Hypo Solution Formula
As the name suggested, chemical formula of hypo solution is Na2S2O3. Sodium thiosulfate (sodium thiosulphate) is a chemical and medication. The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water. It is also called sodium hyposulfite or “hypo”.
Sodium thiosulfate is called hypo as an abbreviated form of one of its historically more common names, hyposulfite of soda. Sodium thiosulfate is an important inorganic salt with several medical uses. It is also called sodium hyposulfite or ‘hypo’. It is used in several pharmaceutical preparations and also has various medical properties.
Frequently Asked Questions – FAQ
Is sodium thiosulfate a salt?
Sodium thiosulfate is an inorganic sodium salt composed of 2:1 mixture of sodium and thiosulfate ions. It has a role as a cyanide poisoning antidote, a nephroprotective agent, and an antimicrobial product.
What is the normality formula?
The normality of a solution is equal to the number of equivalents multiplied by the molarity. Standard(N) = Molarity(M) x equivalent number. It is defined as the gram weight equivalent of the solution per liter.
Is sodium thiosulfate corrosive?
The hydrochloric acid solution is eye and skin corrosive. Via absorption and inhalation, it is mildly toxic. Sodium thiosulfate solution is an irritant to the body’s skin. Sulphur dioxide gas, skin, and eye irritant are produced by the reaction of sodium thiosulfate and hydrochloric acid.
What is the equivalent weight of sodium thiosulphate?
The weight equivalent is the gram molecular weight divided by the number of electrons lost or obtained by each molecule; this is (248.17/1) g for sodium thiosulfate (Na2S2O3.5H2O).
Is sodium thiosulfate an oxidizing agent?
Thiosulfate ion (S2O3-2) is a moderately strong reducing agent used by an indirect procedure in which iodine is an intermediate agent to determine oxidizing agents. Starch irreversibly decomposes in solutions containing high levels of iodine.
Thus, it is evident that sodium thiosulfate is a very important chemical compound in the lives of human beings. To learn more about this compound and its reaction with hydrochloric acid, register with BYJU’S and download the mobile application on your smartphone.

is sodium thiosulphate reacts with mild oxidising agents
Sodium thiosulphate gets oxidized to SO42− and colloidal sulphur [S4O62− is formed]. Click here to learn about the Sodium thiosulphate
Sodium thiosulphate should be used fairly soon after preparation. Explain why this is so
Infusion of sodium thiosulfate (10 percent) may prevent SM toxic manifestations, provided it is administered immediately after exposure and no later than 30 minutes after exposure.