The Sonogashira Coupling was proposed in 1975 by Nobue Hagihara, Yasuo Tohda and Kenkichi Sonogashira in their publication. This reaction is an extension of Cassar and Dieck and Heck reactions, that carry coupling with the use of palladium catalyst. But the Sonogashira reaction uses copper and palladium catalysts continuously.
It is a cross-coupling reaction that is one of the most used reactions for coupling aryl or vinyl halides and is also used in organic synthesis to produce carbon-carbon bonds. It is important in a wide variety of areas and has become essential in the synthesis of compounds with application in material science pharmaceuticals, nanomaterials and natural product chemistry. It can be followed in mild conditions like room temperature to synthesise complex molecules.
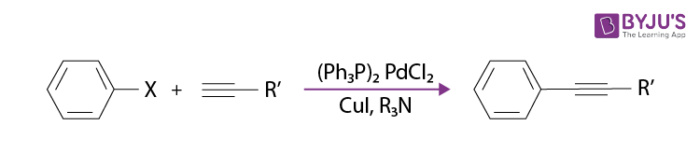
A simple example of the sonogashira coupling reaction is tazarotene synthesising in a treatment of acne and psoriasis, also called as Altinicline.
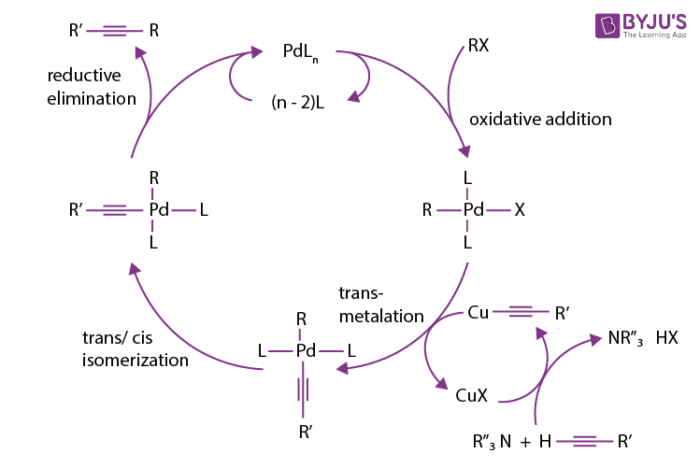
The mechanism of the sonogashira coupling reaction is not completely understood as it is difficult to analysing and isolating of organometallic compounds. These organometallic compounds are present in the reactions as intermediates. However, the mechanism is predicted around the copper cycle and palladium cycle.
The formation of pi-alkyne complex and complex E is based on the presence of base. It makes the proton more acidic on alkyne. While, the compound F reacts with the palladium and regenerates the copper halide.

Comments