Table of Contents
What is Steam Distillation?
Steam Distillation is a separation process for temperature-sensitive substances. It is a particular type of distillation. Another way around is a method for separating miscible liquid bases depending upon their volatilities. For instance aromatic compounds. It plays a vital role in some industrial regions. Here no chemical reaction takes place. It is a physical process.
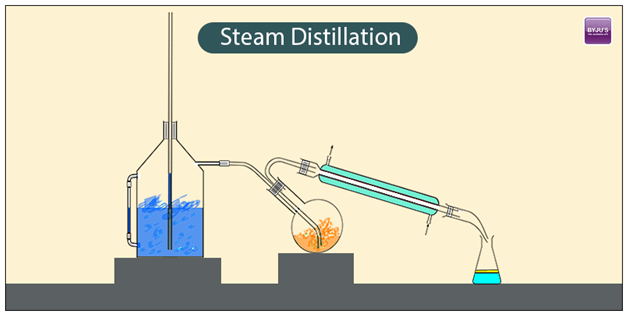
At a very high sustained temperature, few of organic compounds may decompose so it would be difficult to separate them by a simple distillation process at a certain boiling point.
The condensed liquid phase contains vaporized water vapour with required compounds, and they are placed in a condensation flask that is placed nearby. Now distillation takes place at a lower temperature. Steam distillation can be applied in case the substance is very sensitive to heat. Vapours are condensed after the distillation process.
Recommended Videos
Principle of Steam Distillation
Miscibility is a characteristic of a mixture that would mix well in the right proportion leading to the formation of homogeneous mixtures. When such immiscible mixtures are heated and it starts arousing to unmask the surface to a phase that involves the conversion of liquid to vapour phase.
Here vapour pressure is extracted independently by an individual constituent on its own. The Vapour pressure of the system increases consequently, the two immiscible liquids start to boil when the vapour pressure of these liquids out place the atmospheric pressure. Many organic compounds are insoluble in water. They can be purified at an absolute temperature that is below the point where decomposition occurs.
Applications of Steam Distillation
Steam distillation are widely used in the manufacturing of essential oils, for instance perfumes. This method uses a plant material that consists of essential oils. Mainly orange oil is extracted on a large scale in industries using this method.
The application of steam distillation can be found in the production of consumer food products and petroleum industries. They are used in the separation of fatty acids from mixtures.
Frequently Asked Questions – FAQs
What is steam distillation used for?
The procedure is referred to as steam distillation if water is used as one of the immiscible liquids. It is often used to purify liquids that at their usual boiling points decompose. For removing organic compounds from plant pieces, steam distillation is used.
How is steam distillation different from simple distillation?
This behaviour happens because boiling requires a lower vapour pressure, which can be accomplished at a lower temperature. Steam distillation is similar to basic distillation, the only difference being that, along with the substance to be distilled, steam (or water) is used in the distilling jar.
Which compounds are steam volatile?
Distillation that distinguishes the target product from other materials or impurities also purifies volatile organic compounds. Under steam distillation conditions, nitrophenol distils, thus o-Nitrophenol is volatile steam.
What is steam distillation of essential oils?
The most common way to remove aromatic compounds (essential oil) from a plant is steam distillation. The steam moves through the plant material during the steam distillation process. The mixture of hot steam and gentle pressure allows microscopic protective sacs to release the essential oil.
Why is Ortho Nitrophenol steam volatile?
O-Nitrophenol forms an intramolecular H bond whereas molecules of P-Nitrophenol get associated through an intermolecular H bond. During boiling, the strong intermolecular H bonding increases the boiling point but intramolecular H bonding cannot do so. Therefore, O-Nitrophenol is more steam volatile than P-Nitrophenol.

Is Steam distillation is the final step of mineral oil production process?
Mineral oils can be produced from the refined hydrocarbons that are obtained from crude oil via the process of distillation. Steam distillation is one of the methods used to obtain the required product.