Stereochemistry – Viktor Meyer (1848–97) used the term in 1878 to describe the study of stereoisomers. In 1848, Louis Pasteur demonstrated that tartaric acid has optical activity, which is dependent on molecular symmetry, and in 1874, Jacobus H. van’t Hoff and Joseph-Achille Le Bel (1847–1930) independently demonstrated that a molecule with a carbon atom bonded to four different groups has two mirror-image forms.
Stereochemistry is the branch of chemistry that deals with stereoisomers and asymmetric synthesis. For studies on stereochemistry and stereoisomerism of alkaloids, enzymes, antibiotics, and other natural substances, John Cornforth (b. 1917) and Vladimir Prelog (1906–98) received the Nobel Prize in 1975.
|
Definition: Stereochemistry is a branch of chemistry that studies and manipulates the relative spatial arrangement of atoms that make up the structure of molecules. Stereochemistry is the study of stereoisomers, which have the same chemical formula and bonded atom sequence (constitution) but differ in the three-dimensional orientations of their atoms in space. |
Stereochemistry Chemistry Questions with Solutions
Q1: Compounds with different atomic configurations in space but the same atoms bonded to each other are said to as having.
a) stereoisomerism
b) functional group isomerism
c) chain isomerism
d) position isomerism
Answer: a) stereoisomerism
Explanation: Stereoisomer varies from structural isomers, which have the same molecular formula but differ in their bond connections or order. Molecules that are stereoisomers of one other have the same structural isomer by definition.
Q2: Which of the following terms best describes the following pair of molecules?
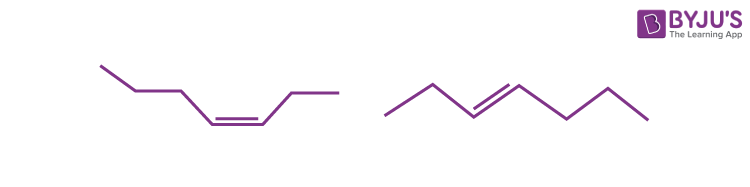
a) Isomers
b) Geometrical isomers
c) Configurational isomers
d) Constitutional isomers
Answer: b) Geometrical isomers
Explanation: These molecules are isomers since they have the same chemical formula (C7H14). However, due to a double bond, the molecules’ spatial orientations differ. And hence, the molecules can best be characterised as geometric isomers.
Q3: Which of the following alkanes has the ability to exhibit optical activity?
a) Neopentane
b) Isopentane
c) 3–Methylpentane
d) 3–Methylhexane
Answer: d) 3–Methylhexane
Explanation: 3–Methylhexane shows the optical activity as shown below:
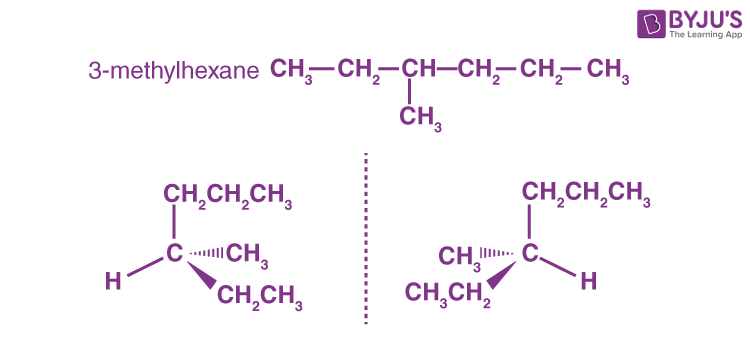
Q4: What is the molecular formula for the stereogenic centre alkane with the smallest molecular weight?
a) C7H16
b) C5H12
c) C6H14
d) C4H10
Answer: a) C7H16
Explanation: Because optical isomerism is only observed in compounds with a chiral carbon, an unsymmetrical alkane can exhibit optical isomerism. Alkanes with 6 and 8 carbons is not the answer. The lowest alkane is 3 methyl hexane, which is a 7 carbon alkane, has a chiral carbon on the third carbon.
Q5: Which of the following statements regarding this compound’s stereochemistry can be deduced?

a) This compound is optically active because the compound contains a centre, plane, or axis of chirality
b) This compound is optically active because it has stereogenic centers that create cis-trans isomers
c) This compound is not optically active since there are no stereogenic centers
d) This compound is not optically active because of its symmetry
Answer: b) This compound is optically active because it has stereogenic centers that create cis-trans isomers
Explanation: Any quaternary or tertiary carbons would be a good area to look for stereogenic centres. There are no quaternary carbons in the benzene ring structure, yet all tertiary carbons are part of it.
Q6: What is meant by Chelate effect?
Answer:
A five or six-membered ring is created when a bidentate or polydentate ligand comprises donor atoms positioned in such a way that when they coordinate with the centre metal ion. The Chelate effect is the name for this process. As a result, the complex’s stability improves. For example, the complex of Ni2+ with ‘+ion’ is more stable than NH3.
Q7: Give the importance of Stereochemistry in association with the Thalidomide Disaster.
Answer:
The arrangement of the atoms in three-dimensional space has a significant impact on the molecule’s properties.
The thalidomide accident in Germany in 1957 is an example of the importance of stereochemistry. The drug thalidomide was first sold as an over-the-counter anti-nausea medication. Pregnant women took it to help with morning sickness.
However, due to the process of metabolism, it was observed that the medicine underwent racemization and generated a mixture of enantiomers in the human body.
One of these enantiomers is suspected of causing genetic harm in developing embryos and resulting in birth abnormalities in children. This is based on the fact that over 5000 kids were born with deformed limbs immediately after thalidomide became available over the counter.
As a result of the medicine’s unexpected side effects, tougher drug regulation rules were enacted (only 40% of the babies born with these deformities survived). The disaster emphasises the significance of stereochemistry.
Q8: There are three chiral centres in a molecule. In comparison to the original molecule, how many stereoisomers of this compound will have different boiling points?
(a) Seven
(b) Two
(c) One
(d) Six
Answer: (d) Six
Explanation: The first step is to figure out how many stereoisomers this molecule has. We may solve using equation 2𝐧, where 𝐧 is the number of chiral centres because the number of stereoisomers is proportional to the number of chiral carbons. We know there are eight stereoisomers for this molecule because there are three chiral centres. It’s important to remember that this figure includes the original molecule.
2𝐧 = 23 = 8
The following step is to compare the various stereoisomers to the original molecule. There will be one enantiomer and six diastereomers in the original molecule. We won’t include this isomer in the final answer because enantiomers have identical physical properties. Diastereomers, on the other hand, differ from the original molecule in terms of physical properties. As a result, the boiling temperatures of six stereoisomers will differ from the original molecule.
Q9: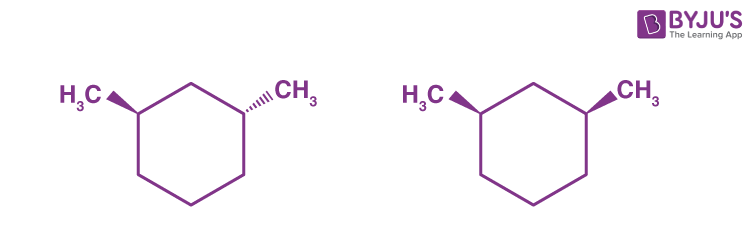
The given molecules are __________.
(a) constitutional isomers
(b) conformers
(c) stereoisomers
(d) identical
(e) None of these
Answer: (c) stereoisomers
Explanation: Around a single stereocenter, stereoisomers have different orientations. Stereoisomers are molecules that have the same shape. These molecules are epimers, which means that they differ only at one stereocenter.
The molecular formula of constitutional isomers is the same, but their structures are different. Around a single bond, conformers rotate in different directions. Clearly, the molecules are not identical.
Q10: Explain the differences between cis and trans decalin’s stability and physical qualities.
Answer:
The chair form of decalin’s six-membered rings, like those of cyclohexane, is believed to be the most stable (Figure). Two chairs, on the other hand, can be joined in one of two ways. The hydrogens at the ring junction might be on the same side of the molecule (cis-decalin) or on opposite sides (trans-decalin).
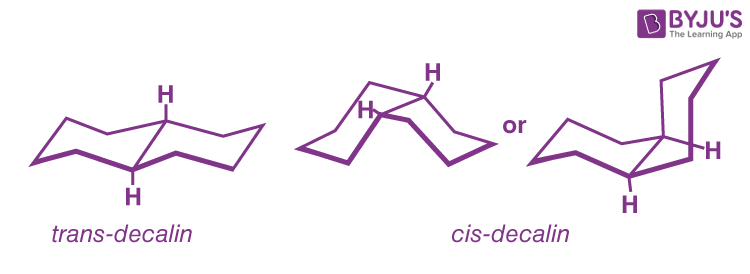
Trans-decalin is produced when the two rings are linked by two equatorial-type bonds, whereas cis-decalin is produced by an axial-equatorial union. Both isomers are known, and the trans isomer is roughly 2kcal mol-1 more stable than the cis isomer, owing to unfavourable nonbonded interactions within the concave area of cis-decalin.
Q11: Define Chiral catalyst. What is a Chiral reagent?
Answer:
Most of the chiral catalysts, also known as asymmetric catalysts, are made up of transition metals and chiral ligands. These catalysts are very efficient even at low substrate-to-catalyst ratios, making them great synthetic tools at industrial scales. Metal-free chiral catalysts, such as the MacMillan imidazolidinone organocatalysts or proline-based organocatalysts, are also available.
A chiral derivatizing agent (CDA), also known as a chiral resolving reagent, is a chiral auxiliary that is used to transform a mixture of enantiomers into diastereomers so that the proportions of each enantiomer present in the mixture can be determined. Spectroscopy or chromatography can be used for analysis. With the popularity of chiral HPLC, the use of chiral derivatizing agents has reduced. Chiral derivatization is also utilised for chiral resolution, which is the actual physical separation of the enantiomers, in addition to analysis.
Q12: What is Positional Isomerism?
Answer:
Positional isomers are constitutional isomers with identical carbon skeleton and functional groups but differ in where the functional groups are located on or in the carbon chain.
Propyl bromide (1) and isopropyl bromide (2) are constitutional isomers. They have the same carbon skeleton (C𑁋C𑁋C).

They both contain a bromine atom as a functional group. The location of the bromine atom on the carbon chain differs between 1 and 2. In 1, the bromine atom is attached to a terminal carbon atom, whereas in 2, it is attached to an internal carbon atom. And hence, 1 and 2 are positional isomers.
Q13: What is Chiral Synthesis?
Answer:
Chiral synthesis (also known as asymmetric synthesis or enantioselective synthesis) is a type of chemical synthesis. It is described by IUPAC as a chemical reaction (or reaction sequence) that produces stereoisomeric (enantiomeric or diastereoisomeric) products in uneven proportions when one or more new components of chirality are produced in a substrate molecule.
Chiral synthesis is an important process in modern chemistry, especially in the field of medicines, because a molecule’s different enantiomers or diastereomers often have different biological activities.
Q14: Give the difference between Stereospecific and Stereoselective Reactions.
Answer:
Chemical reactions involving organic compounds that produce products with different atomic configurations are referred to be stereospecific and stereoselective. The main difference between stereospecific and stereoselective reactions is that a stereospecific reaction produces only one product, whereas a stereoselective reaction produces several.
Definition
Stereospecific Reactions: A stereospecific reaction is one in which the reactant’s stereochemistry totally determines the stereochemistry of the product with no other options.
Stereoselective Reactions: A stereoselective reaction is one in which there are multiple pathways to choose from, but the product stereoisomer is formed due to the reaction pathway being more favourable than the others.
Effects
Stereospecific Reactions: The stereochemistry of the reactant determines the final product of a stereospecific reaction.
Stereoselective Reactions: Differences in steric effects (the presence of bulky groups causes steric hindrance) and electronic effects determine the reaction pathway’s selectivity.
Number of Products
Stereospecific Reactions: From a specific reactant, a stereospecific reaction yields a specific product.
Stereoselective Reactions: Multiple products can develop from a stereoselective reaction.
Q15: Briefly describe the types of Stereoisomers.
Answer:
- Enantiomers: An enantiomer, also known as an optical isomer, antipode, or optical antipode, is one of two stereoisomers that are mirror images of each other but are non-superposable (not identical), similar to how one’s left and right hands are mirror images of each other but are not identical simply by reorientation.
The sedative thalidomide, which was sold in a number of nations around the world from 1957 to 1961, is an example of such an enantiomer. The antidepressants escitalopram and citalopram are other examples.
- Diastereomers: Diastereomers (also known as diastereoisomers) are a form of stereoisomer. Diastereomers are characterised as non-image non-identical stereoisomers. As a result, they happen when two or more stereoisomers of the same compound have distinct configurations at one or more (but not all) of the equivalent (related) stereocenters and aren’t mirror reflections of each other.
Epimers are diastereoisomers that differ from each other at only one stereocenter. Each stereocenter generates two different configurations, resulting in a two-fold increase in the number of stereoisomers.
- Atropisomerism: Atropisomers are stereoisomers that develop as a result of hindered rotation around a single bond when energy differences caused by steric strain or other factors produce a high enough barrier to rotation to allow distinct conformers to be isolated.
Many atropisomers can be found in nature, and some of them can be used in medicine development. Mastigophorene A, a natural substance, has been discovered to help in nerve growth.
- Cis–trans isomerism: Cis–trans isomerism, also known as geometric or configurational isomerism, in chemistry, refers to the spatial arrangement of atoms within molecules. The Latin prefixes “cis” and “trans” mean “this side of” and “the other side of,” respectively.
Cis denotes that the functional groups (substituents) are on the same side of a plane, while trans expresses that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that are pairs of molecules that have the same formula but distinct functional groups in three-dimensional space.
- Conformational isomerism: Conformational isomerism is a type of stereoisomerism in which isomers can be changed by rotating them around formally single bonds. While any two atomic configurations in a molecule that differ by rotation about single bonds are called distinct conformations, conformations that correspond to local minima on the potential energy surface are called conformational isomers or conformers.
Practise Questions on Stereochemistry
Q1: What is the stereochemical relationship of the two compounds below?
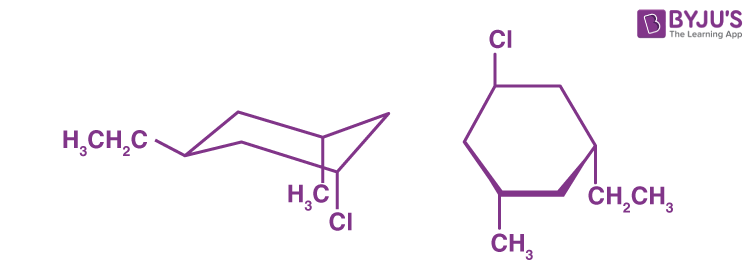
a) Geometrical isomers
b) Enantiomers
c) Diastereomers
d) Identical
Q2: In the following molecule, what is the relationship between the two groups?
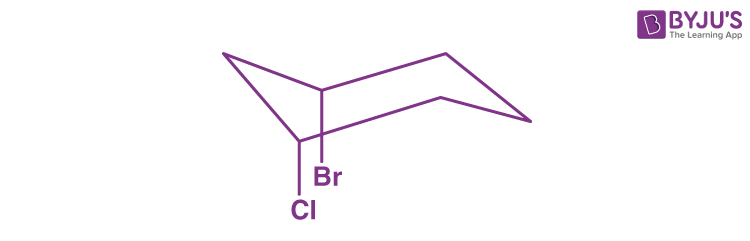
a) They are equatorial to one another
b) They are axial to one another
c) They are cis to one another
d) They are trans to one another
Q3: What is Regiochemistry and stereochemistry?
Q4: Indicate the types of isomerism exhibited by the complex [Co(NH3)5 (NO2)] (NO3)2. (At. no. Co = 27).
Q5: The molecules shown below are best described as __________.
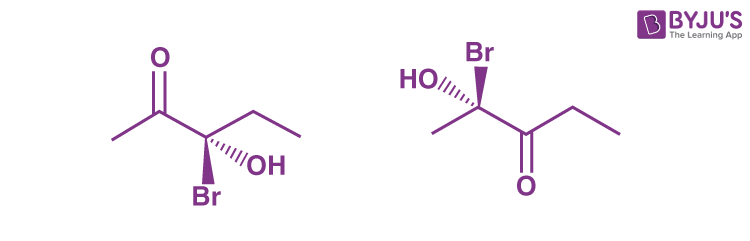
(a) diastereomers
(b) epimers
(c) isomers
(d) enantiomers
Click the PDF to check the answers for Practice Questions.
Download PDF
Recommended Videos
Stereochemistry of E2 mechanism
Conformational Stereoisomerism

Comments