What is Acetylene?
Acetylene is the simplest member of the alkyne family. Alkynes are unsaturated hydrocarbons in which a carbon-carbon triple bond exists between the two carbon atoms.
Quantum mechanics helps us a great deal to study the structure of different molecules found in nature. The concept of chemical bonding in combination with quantum mechanics has revealed numerous information about various organic and inorganic compounds that are essential for life. This article deals with the structure of a special class of organic compounds known as alkynes.
Structure of Acetylene – The Triple Bonds
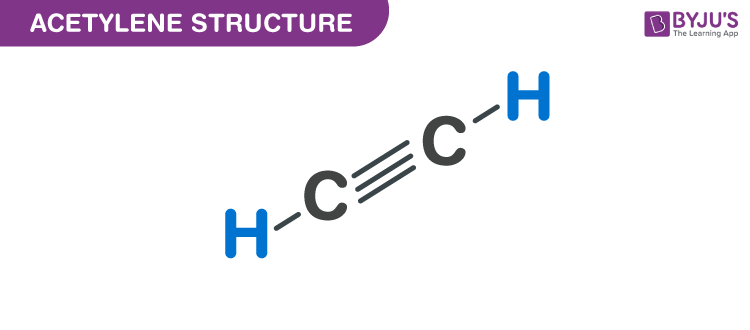
Acetylene Structure
What is a triple bond?
When three pairs of electrons are shared between two carbon atoms, a triple bond is formed between the two carbon atoms. The distinguishing features of alkynes from the other hydrocarbons are the triple bond which exists between the carbon atoms.
The triple-bonded carbon atoms of acetylene are sp hybridized. Sigma bonds between the atoms of carbon are obtained by a head-on overlapping of the two hybridized sp orbitals. The leftover orbitals of the carbon atoms overlap with each other along the internuclear axis in the 1s orbital of each hydrogen atom which results in the formation of one C-H sigma bond and 2 weaker pi bonds. These bonds are formed due s orbital overlapping. Each carbon atom uses only one of its three p-orbitals. Hence the two remaining p orbitals are occupied by a single electron. As it is the same with the other carbon atom, this permits the pairing of electrons which results in the formation of 2 pi bonds. The carbon-carbon triple bond is made stronger by the presence of one sigma bond.
Acetylene is a linear molecule with carbon-carbon distance of 1.21 Ao. The C-H distance in acetylene is1.08 Å shorter than alkenes due to the fact that sp hybridized carbon has more s character than the sp2 hybridized carbon. To explore more alkynes and other hydrocarbons please visit the YouTube channel of Byju’s – The Learning App.

Thanks to the byju’s team.