What is Tannic acid?
Tannic acid is a type of polyphenol and is also called Acidum tannicum or Gallotannic acid.
Tannic acid has numerous phenol groups and hence is a weak acid. It is obtained naturally from tara pods, Quercus infectoria, gallnuts from Rhus semialata, Sicilian Sumac leaves. The chemical formula of Tannic acid is C76H52O46.
The two main types of Acidum tannicum are Condensed tannins and Hydrolyzable tannins. The word tannin is derived from the Celtic word for Oaktree which was once used for leather processing.
Table of Contents
- Properties of Tannic acid – C76H52O46
- Structure of Tannic acid (C76H52O46)
- Uses of Tannic acid (C76H52O46)
- Health hazards
- Frequently Asked Questions
Properties of Tannic acid – C76H52O46
| Tannic acid | C76H52O46 |
| Molecular Weight of Tannic acid | 1701.19 g/mol |
| Density of Tannic acid | 2.12 g/cm3 |
| Melting Point of Tannic acid | decomposes above 200 °C |
| pKa of Tannic acid | ca. 6 |
Structure of Tannic acid (C76H52O46)
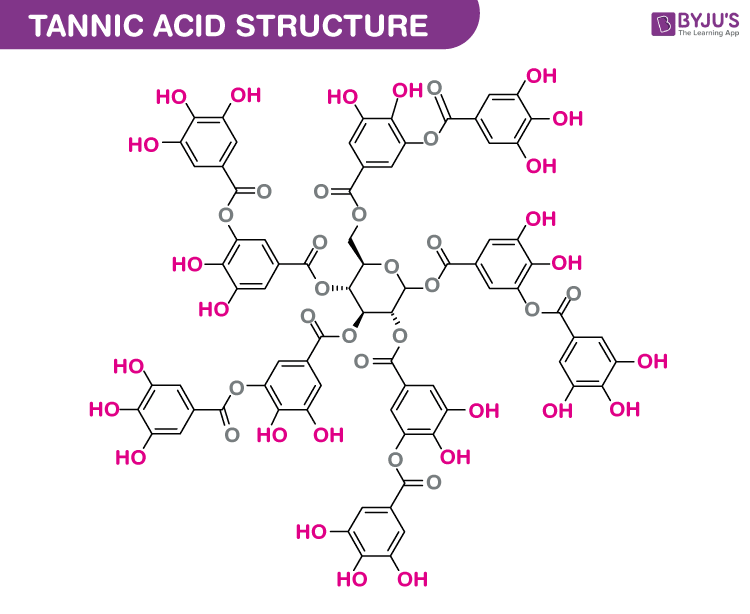
Structure of Tannic acid (C76H52O46)
Uses of Tannic acid (C76H52O46)
- Tannic acid in the production of albumin tannate which is used as an antidiarrheal agent.
- It has wide application in the food industry as it is used as a taste enhancer, colour stabilizer, and clarifying agent.
- It is used in the conservation of ferrous metal objects to inhibit corrosion.
- It is used in the dyeing process for cellulose fibres.
- It is used to impart anti-staining properties to polyamide carpets or yarn.
- It is used as a coagulant in the manufacturing of rubber.
- It is used as a reagent in analytical chemistry.
- It is used with a mixture of albumin and gelatin to manufacture tortoiseshell.
Health hazards
Tannic acid can damage the eye, respiratory tract, gastrointestinal tract, and skin. It causes irritation, pain, redness, and blurred vision. When it is absorbed through the skin, it may cause redness and irritation. When ingested it can cause nausea, diarrhoea, and vomiting.
Frequently Asked Questions
Where is tannic acid found naturally?
Tannic acid is present in nutgalls, and arboreal swelling is caused by parasitic wasps. The most common occurrence of tannic acid, however, is in the twigs of certain trees, specifically Chestnut and Oak trees.
What is the formula of tannic acid?
The chemical formula for commercial tannic acid is sometimes given as C76H52O46, which corresponds to decagalloyl glucose, but it is, in reality, a mixture of polygalloyl glucose or polygalloyl quinic acid esters with galloyl molecules varying from 2 to 12 depending on the source of the plant.
Is tannic acid good for health?
Tannic acid has astringent, anti-bacterial, and anti-enzymatic properties. Tannic acid intake has induced constipation, and can be used to treat diarrhoea (without fever or inflammation). Tannic acid is useful for its anti-oxidant and anti-mutagenic effects.
What is tannin used for?
Tannins are used in painting, in addition to tanning leather, as mordants in dyeing, clarifying wine and beer by precipitating proteins from them, and as astringents in medicine. Tannins are commonly found in the bark of trees, wood, leaves, buds, stems, fruits, seeds, roots, and galls of plants.
What is the difference between tannin and tannic acid?
Tannic acid is a special kind of tannin, a kind of polyphenol. The low acidity (pKa at around 6) is due to the structure’s various phenol groups. While tannic acid is a different form of tannin (plant polyphenol) the two words are often used interchangeably.
Learn more about the physical and chemical properties of Tannic acid (C76H52O46) from the experts at BYJU’S.

Comments