Table of Contents
Enzyme Catalysis:
Catalysis is a phenomenon in which the rate of the reaction is altered with the help of a substance called a catalyst (the catalyst does not participate in the reaction; its concentration and composition remain unchanged). The substance used to change the rate of the reaction is called a catalyst. Enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical reactions in plants and animals. The catalysis in which enzymes act as a catalyst is called enzyme catalysis.
Enzymes are complex compounds containing nitrogen. Animals and plants produce these compounds naturally in their bodies. Enzymes are proteins which have high molecular mass and form a heterogeneous mixture when dissolved in water. These proteins act very efficiently and are responsible for various reactions which occur in the body of living beings.
Characteristics of enzyme catalysis:
- A single molecule of the enzyme catalyst can transform up to a million molecules of the reactant per second. Hence, enzyme catalysts are said to be highly efficient.
- These biochemical catalysts are unique to certain types of reactions, i.e. the same catalyst cannot be used in more than one reaction.
- The effectiveness of a catalyst is maximum at its optimum temperature. The activity of the biochemical catalysts declines at either side of the optimum temperature.
- Biochemical catalysis is dependent upon the pH of the solution. A catalyst works best at an optimum pH which ranges between 5-7 pH values.
- The activity of the enzymes usually increases in the presence of a coenzyme or an activator such as Na+, Co2+ The rate of the reaction increases due to the presence of a weak bond which exists between the enzyme and a metal ion.
Mechanism of an enzyme catalyst:
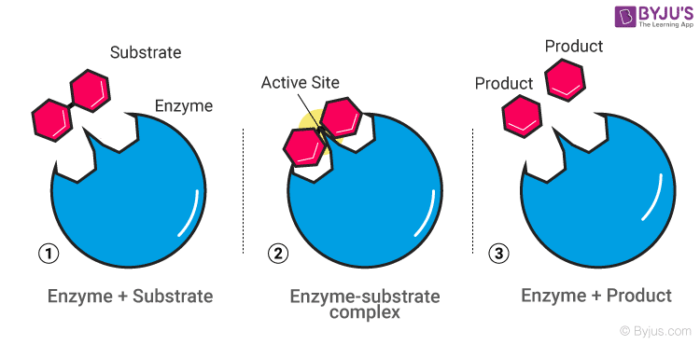
Hence this happens in two steps:
Step1: Combining of enzyme and the reactant
Step 2: Disintegration of the complex molecule to give the product
Interesting concepts in chemistry can be studied with the help of chemistry apps. To learn more about enzyme catalysis, register with BYJU’S and download our app.

Comments