Table of Contents
- What is Thiol?
- Properties of Thiol
- Reactions of Thiol
- Thioxanthates
- Metal ion complexation
- Redox Reactions of Thiols
- S-alkylation
- Acidity
What is Thiol?
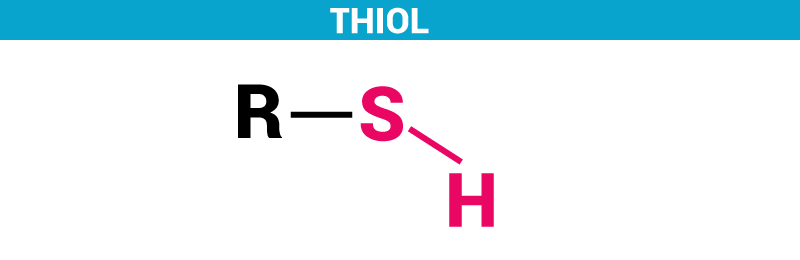
Thiols can be defined as a sulfur analogue of alcohols. In a simpler way, it is an organic compound consisting of compounds with a sulfur atom. It is also referred to as mercaptan. It consists of sulfhydryl group, i.e., Thiol = R-SH.
Alcohols and thiols share some similarities i.e. sulfur emergers as a larger element compared to that of oxygen, and the length of the C–S bond is more than that of the C–O bond. The hydrogen bonding between the thiol groups is much weaker in liquids or solids mainly because of the cohesive force. They exhibit a lower dipole moment than alcohol.
Properties of Thiol
Many thiols consist of odours that are usually strong which may resemble the scent of onions and garlic. Thiols possessing a low molecular weight consist of a repulsive and strong smell. For example, consider skunks consisting of the lower molecular weight of thiol and it is easily detectable by humans. It is also responsible for some sort of wine faults that have resulted due to some unintended reactions caused between sulphur and yeast.
Not all kinds of thiols hold bad odour; there are many other thiols with a pleasant smell. For example, furan-2-yl methanethiol is responsible for the beautiful aroma of roasted coffee, and monoterpenoid thiol is responsible for the mesmerizing scent of a grapefruit. One can find it only at low concentration levels.
Thiols are less likely soluble in water. Thioether functional groups possess similar boiling points and solubility features, well this does not hold true for alcohols. Volatile thiols can be easily identified due to their distinctive odours.
Reactions of Thiol
Many reactions are exhibited by thiols similar to that of hydroxyl compounds involving the formation of sulphides and thioesters. Oxidation would affect the sulphur atom in thiols, whereas in alcohol it would lead to the generation of a new product where it changes the oxidation state of a carbon atom.
Thioxanthates
Thiolates react with carbon disulphide to produce thioxanthate. Thioxanthates are organic compounds with the formula RSCS2X. If X is an alkali metal, then thioxanthate is a salt. IF X is a transition metal, then thioxanthate is a ligand, and if X is an organic group, then the compounds are called thioxanthate esters.
Metal ion complexation
Thiolates form transition metal thiolate complexes with metal ions.
Redox
Thiols readily oxidize through reagent iodine, in the presence of a base to produce organic sulphide. The redox reaction of thiol is mentioned below.
2 R–SH + Br2 → R–S–S–R + 2 HBr
Reagents such as hydrogen peroxide or sodium hypochlorite can also produce sulfonic acids.
R–SH + 3 H2O2 → RSO3H + 3 H2O
In the presence of a catalyst, oxidation can be affected by oxygen.
2 R–SH + 1⁄2 O2 → RS–SR + H2O
Participation of thiols in thiol-disulfide exchange.
RS–SR + 2 R′SH → 2 RSH + R′S–SR′
S-alkylation
Thiols are alkylated to give thioethers.
RSH + R′Br + B → RSR′ + [HB]Br
Acidity
Thiols are more acidic in nature. Thiolate is a conjugate base of thiol. It is obtained from thiol on treatment with alkali metal hydroxides.
Frequently Asked Questions – FAQs
What are thiols?
What is thiol used for?
Are thiols weak or strong?
Among thiol and alcohol, which is more acidic?
What are thioxanthates?
Stay tuned with BYJU’S to learn more interesting topics in Chemistry. Also, get various engaging video lessons to learn more effectively.

Comments