Thomson model Introduction
Thomson atomic model was proposed by William Thomson in the year 1900. This model explained the description of an inner structure of the atom theoretically. It was strongly supported by Sir Joseph Thomson, who had discovered the electron earlier.
During cathode ray tube experiment, a negatively charged particle was discovered by J.J. Thomson. This experiment took place in the year 1897. Cathode ray tube is a vacuum tube. The negative particle was called an electron.
Table of Contents
Thomson assumed that an electron is two thousand times lighter than a proton and believed that an atom is made up of thousands of electrons. In this atomic structure model, he considered atoms surrounded by a cloud having positive as well as negative charges. The demonstration of the ionization of air by X-ray was also done by him together with Rutherford. They were the first to demonstrate it. Thomson’s model of an atom is similar to a plum pudding.
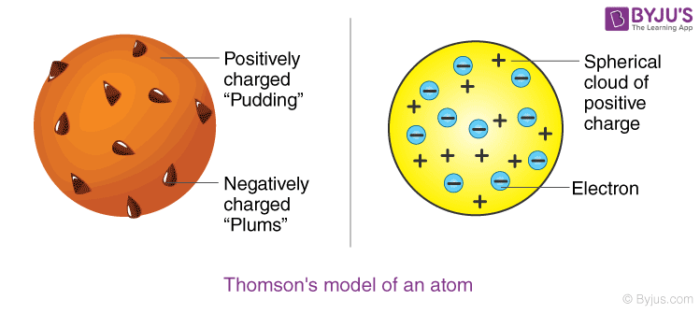
Postulates of Thomson’s atomic model
Postulate 1: An atom consists of a positively charged sphere with electrons embedded in it
Postulate 2: An atom as a whole is electrically neutral because the negative and positive charges are equal in magnitude
Thomson atomic model is compared to watermelon. Where he considered:
- Watermelon seeds as negatively charged particles
- The red part of the watermelon as positively charged
Limitations of Thomson’s atomic model
- It failed to explain the stability of an atom because his model of atom failed to explain how a positive charge holds the negatively charged electrons in an atom. Therefore, This theory also failed to account for the position of the nucleus in an atom
- Thomson’s model failed to explain the scattering of alpha particles by thin metal foils
- No experimental evidence in its support
Although Thomson’s model was not an accurate model to account for the atomic structure, it proved to be the base for the development of other atomic models. Find the atomic structure pdf here. The study of the atom and its structure has paved the way for numerous inventions that have played a significant role in the development of humankind. To follow more download BYJU’S – the learning app.
Recommended Videos

Frequently Asked Questions – FAQs
Why was Thomson’s atomic model discarded?
It was discarded because he was unable to precisely account for the stability of the atom. He proposed that electrons are distributed in the atom in the same way that seeds are distributed in a watermelon or dry fruits are distributed in a Christmas pudding.
What is the important feature of Thomson atomic model?
The following are the characteristics of the Thomson model of an atom:
(i) An atom is made up of a positively charged sphere in which electrons are embedded.
(ii) The magnitudes of the negative and positive charges are the same. As a result, the atom is electrically neutral as a whole.
Which model best explains the atom’s neutrality?
According to Thomson’s atomic model, an atom is made up of a positively charged sphere into which negatively charged electrons are implanted. Because electrons and protons have the same magnitude, an atom as a whole is electrically neutral.
To which fruit has Thomson’s model been compared to?
The model was compared to a watermelon because the red edible part of a watermelon was compared to the sphere with a positive charge, and the black seeds filling the watermelon resembled the electrons inside the sphere.
What was missing from Thomson’s atomic model?
His model lacked a nucleus, protons, and neutrons.

nice material for studies