Nitric acid is part of the inorganic acids. This acid is a colourless fuming liquid when pure but turns yellowish due to the collection of nitrogen oxides. It is extremely corrosive and toxic. Nitric acid is soluble in water and it is also a strong oxidizing agent. It reacts with metals, oxides, and hydroxides, forming nitrate salts.
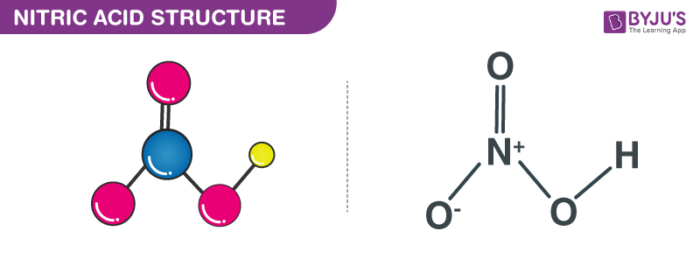
Nitric acid also called the spirit of nitre or aqua fortis comes with the molecular formula of HNO3 which consists of three oxygen atoms, one nitrogen atom and one hydrogen atom. Nitric acid is usually obtained by the oxidation of ammonia. It is mainly done using the Ostwald process. Nitric acid is also formed by treating sodium nitrate with sulphuric acid. But here, it is obtained in small quantities. Nitric acid is also a component of acid rain.
Nitric Acid Uses
Like ammonia, one of the main uses of nitric acid is in the preparation of fertilizers. Nitric acid is also a good oxidizing agent and is used for manufacturing other inorganic compounds. However, this chemical compound has a lot of other uses. It finds its applications in industries as well as in our everyday life. Let’s look at some of them.
- Industrial Uses of Nitric Acid
- Nitric Acid in Fertilizers
- Nitric Acid in Daily Life
Industrial Uses of Nitric Acid
Nitric acid is the building block chemical for the production of many other chemical compounds. It is used in manufacturing several types of polymers like polyamides and polyurethane. Nitric acid is also commonly used as rocket propellants in the aerospace industry. It is also used for manufacturing nitrogen-based compounds like nylon as well as most of the explosives like trinitrotoluene (T.N.T.), nitroglycerin, amongst others.
Other uses include, production of nitrate salts, making dyes, coal tar products and drugs. It is also used mostly for the purification of precious metals like platinum, gold, and silver.
Nitric Acid in Fertilizers
In fertilizer production, Nitric acid is used for manufacturing different types of nitrogenous fertilizers like calcium nitrate, ammonium nitrate, etc. Nitric acid is a key component which is also a by-product of ammonia.
Uses in Daily Life
The most common use of nitric acid is found in schools where it is often utilized as a laboratory reagent. Diluted nitric acid is used in woodworks to fabricate maple and pine wood and make them look aged. It is also used in food industries and helps in cleaning food and equipments, etc.
More significantly, nitric acid is also used to spot test alkaloids like LSD. Another test known as the colourimetric test which requires nitric acid is used to differentiate between morphine and heroin.
These are some popular uses of nitric acid. To know more about the properties, and the structure of acids and bases you can keep visiting BYJU’S or download our app for interesting content and a learning experience.

Comments