What is Hydroboration?
Hydroboration is the process wherein the hydrogen boron bond is added to a double bond between either carbon and carbon or carbon and nitrogen. It can also be performed on a carbon-carbon triple bond. Hydroboration is quite useful in the synthesis of some organic compounds.
Hydroboration can also be used to produce organoborane compounds which are chemical compounds which have carbon-boron bonds and are derivatives of BH3. These organoboranes can be reacted with some reagents to give alkyl halides, alcohols, and amines which are quite useful. Alcohols are produced from the oxidation of the organoboranes with the help of hydrogen peroxide.
Table of Contents
- Boron-Carbon Bond Properties
- Process of Adding H-B Bond to C=C Bond
- Reactions with Substituted Alkenes
- Recommended Videos
Boron-Carbon Bond Properties
The electronegativity of carbon is 2.55 and the electronegativity of boron is 2.04. This is why the carbon-boron bond has a relatively low polarity. The low polarity of the B-C bond makes the alkyl boron compounds quite stable. On the other hand, alkyl boron compounds are also easily oxidizable.
Due to the low electronegativity of boron, it tends to form electron-deficient compounds. An example of the type of compounds produced by boron is triorganoboranes. The carbon boron bond gets some double bond characteristics from the donation of electrons by the vinyl and aryl groups. This causes it to be less electrophilic.
Process of Adding H-B Bond to C=C Bond
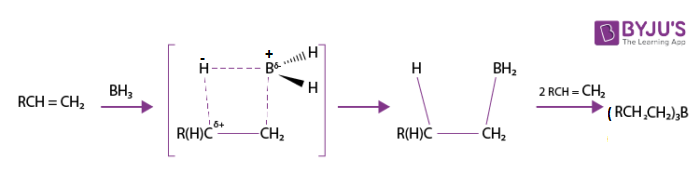
In the hydroboration process, it is observed that hydrogen is added to the most substituted carbon of the double bond. Therefore, hydroboration can be considered an anti-Markovnikov process. This process proceeds via a four-membered transition state. In this state, there is a same-face addition of the boron and hydrogen atoms on the double bond. The carbon boron bond is formed slightly faster than the carbon-hydrogen bond.
Therefore, the boron gains a partially negative charge whereas the more substituted carbon gains a partially positive charge in the four-membered transition state. An example explaining the hydroboration of a given terminal alkene to a trialkyl borane via the four-membered transition state is shown below.
Reactions with Substituted Alkenes
The boron atom is mostly placed on the carbon which is less substituted in trisubstituted alkenes. An example of this reaction is given below.
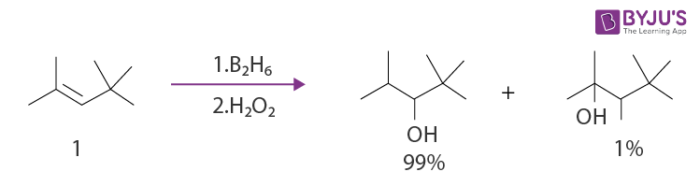
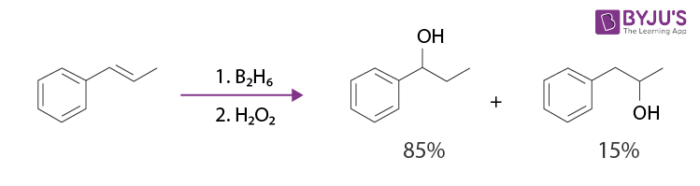
When 1,2-disubstituted alkenes are subjected to hydroboration, two organoboranes in a mixture are produced. Examples of these reactions can include the hydroboration-oxidation reaction of (E)-prop-1-en-1-ylbenzene.
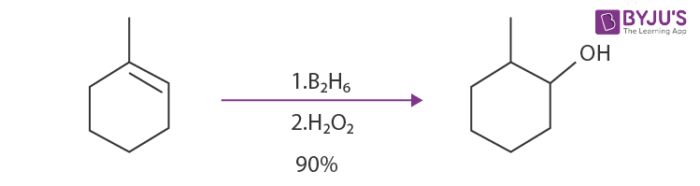
Another example would be the hydroboration-oxidation reaction of 1-methyl-cyclohex-1-ene.
Thus, the H-B bond can be added to the carbon-carbon double bond or the carbon-nitrogen double bond and also on a carbon-carbon triple bond.
Recommended Videos
Oxidation of Alkenes

Comments