Chloroauric acid is an inorganic compound. It is also known as tetrachloroauric acid, auric acid, brown gold chloride and gold trichloride. The property value of hydrogen bond donor and hydrogen bond acceptor are 1 and 1 respectively.
Following is the table of formulas of chloroauric acid:
| Molecular formula | HAuCl4 |
| Linear formula | AuCl4H |
| The Simplified molecular-input line-entry system (SMILES) | [H+].Cl[Au-](Cl)(Cl)Cl |
Structure Of Chloroauric Acid
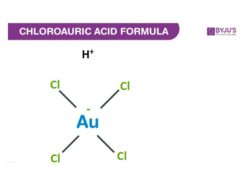
Properties Of Chloroauric Acid
| IUPAC name | hydrontetrachlorogold(1-) |
| Molecular formula | HAuCl4 |
| Molecular mass | 339.785 g/mol (anhydrous)
393.833 g/mol (trihydrate) 411.85 g/mol (tetrahydrate) |
| Density | 3.9 g/cm3
2.89 g/cm3 |
| Melting point | 254℃ |
| Appearance | Yellow-orange needle-like crystals |
Applications Of Chloroauric Acid
- Chloroauric acid is used for the purification of gold by the electrolysis process.
- Gold Ruby’ or the cranberry glass gets its red colour by using chloroauric acid.
Safety Measure
- Health hazards related to chloroauric acid are tissue destruction because of prolonged contact with the skin and when inhaled can cause irritation to mucous membranes.
To learn more about concepts related to Chemistry, stay tuned with BYJU’S.
Comments