Cobalt (II) sulfate formula, also named as Cobalt sulfate formula or Cobaltous sulfate formula is discussed in this article. It is a metallic salt and a toxic inorganic compound. The molecular or chemical formula of Cobalt (II) sulfate is CoSO4.
Cobaltous sulfate is a red to rose-pink solid. It has no odour and sinks as well as mixes well in water. It is insoluble in ammonia. It is widely used in the electrochemical industries, as a colouring agent, as a supplement for Vitamin B12deficiency, as a drier in paints and inks, and in storage batteries.
Cobalt (II) sulfate Formula Structure
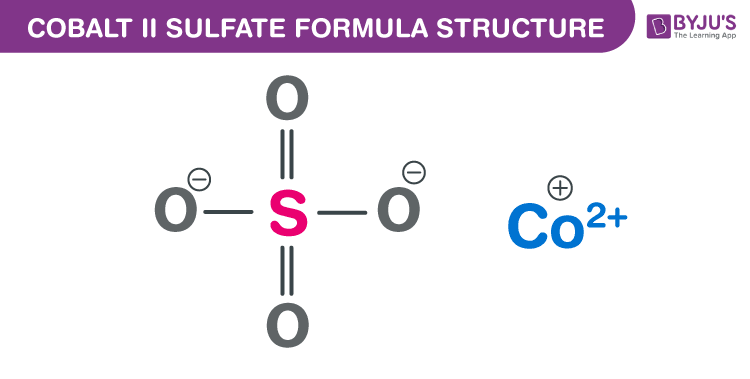
Properties Of Cobalt (II) sulfate Formula
| Chemical formula | CoSO4∙(H2O)7 |
| Molecular weight | 154.996 g/mol (anhydrous)
173.01 g/mol (monohydrate) 263.08 g/mol (hexahydrate) 281.103 g/mol (heptahydrate) |
| Density | 3.71 g/cm3 (anhydrous)
3.075 g/cm3 (monohydrate) 2.019 g/cm3 (hexahydrate) 1.948 g/cm3 (heptahydrate) |
| Appearance | Reddish ((anhydrous, monohydrate)
Pink salt (hexahydrate) |
| Melting point | 735 °C (anhydrous) |
Safety Measures
- When exposed to this compound it causes severe irritation in the respiratory tract, skin, and eyes.
- It affects the lungs, kidneys, and heart. It is anticipated to be a human carcinogen.
To learn more about Cobalt (II) sulfate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Cobaltous sulfate for free.
Comments