Hexane is an alkane consisting of six carbon atoms with a chemical formula C6H14. The term may refer to either any of the five structural isomers with that formula or to a mixture of them. Hexane is a significant constituent of gasoline. Hexanes are chiefly obtained by refining crude oil. The specific composition of the fraction primarily depends on the source of the oil and the constraints of the refining. In this short piece of article, let us learn more about the hexane formula, its properties and chemical structure along with its uses.
Properties of Hexane
| Hexane Properties | |
| Name | Hexane |
| Appearance | Clear colourless liquid |
| Molecular Formula | C6H14 |
| Melting Point | –96°C to –94°C |
| Boiling Point | 68.5°C to 69.1°C |
| Density | 0.6066 g mL–1 |
| Molar Mass | 86.18 g/mol |
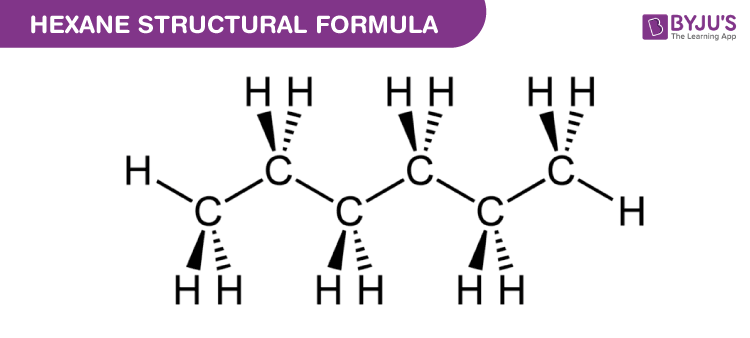
Chemical Structure of Hexane
Hexane Uses
- Used in leather products, formulation of glues for shoes and roofing
- Used to extract oil and grease contaminants from soil and water for analysis
- Used in chromatography as a non-polar solvent
To learn more about such chemistry topics register to BYJU’S now!
Comments