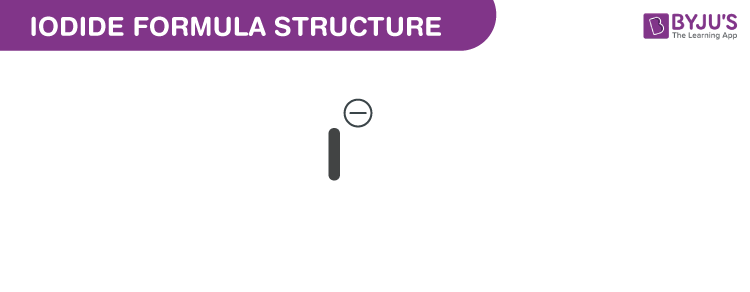
Iodide formula, also known as Iodide ion formula or Iodide anion formula is explained in this article. Compounds containing iodine with an oxidation state -1 are iodides. It is one of the greatest monatomic anions. The assigned radius is approximately 206 picometers. It is The chemical or molecular formula of Iodide I−.
The natural crystalline silver iodide – Iodargyrite is the most common iodide mineral lately known. Iodide anions can be found combined copper, lead, and mercury. These salts are mild reducing agents and can react with oxygen to produce iodine. A reducing agent is also called as an antioxidant in chemical terminology. Iodide salts are usually soluble in water.
Iodide Formula Structure
Properties Of Iodide
| Chemical formula | I− |
| Molecular weight | 126.90447 g/mol |
| Conjugate Acid | Hydrogen iodide |
| Functions as | an antioxidant |
| Std molar entropy | −7.2 °C |
Iodide in its liquid form dissolves iodine better when compared to pure water due to the formation of complex ions.
To learn more about Iodide formula from the expert faculties at BYJU’S, register now! You can also download chemistry study materials such as syllabus, textbooks, practice papers, etc. for free.
Comments