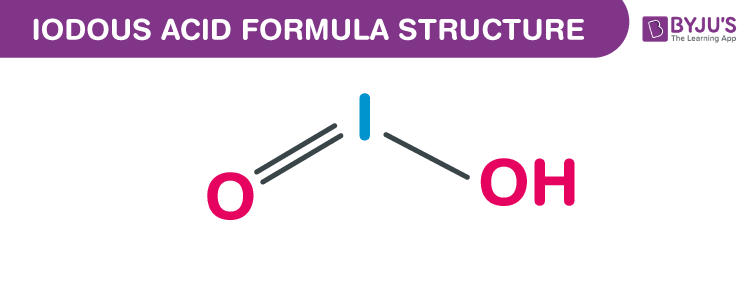
Iodous acid formula, also named as Hypoiodic acid formula or Iodic acid formula is discussed in this article. Hypoiodic acid is a conjugate acid of an iodite and an iodine oxoacid. The molecular or chemical formula of Iodous acid is HIO2.
The salts of this chemical compound are named iodites. These are extremely unstable, observed but never isolated. They disproportionate quickly to molecular iodates and iodine. Its molecular formula is 159.911 g/mol.
Iodous acid Formula Structure
Properties Of Iodous acid Formula
| Chemical formula | HIO2 |
| Molecular weight | 159.911 g/mol |
| Hydrogen bond donor | 1 |
| Conjugate base | Iodite |
| Complexity | 10.3 |
The number of hydrogen bond donor is one whereas the number of hydrogen bond acceptors are two. The monoisotopic mass is equal to 159.902 g/mol.
To learn more about Iodous acid formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Iodic acid for free.
Comments