What Is Azeotropic Mixture?
An azeotropic mixture is a mixture of substances that has the same concentration of vapour and fluid phases. It is basically a mixture that contains two or more liquids. A zeotropic mixture basically has constant or the same boiling points, and the mixture’s vapour will also have the same composition as the liquid.
Download Complete Chapter Notes of Solutions
Download Now
Normally, we use distillation to isolate materials as the ideal solutions, with one part more volatile than the other. However, in an azeotropic mixture, since the vapour and fluid concentrations will be the same, this approach will prevent their separation.
Two, three and more azeotropes can be either uniform or heterogeneous (more than a phase). Azeotrope usually happens when a mixture is heated in order to produce vapour with the same liquid composition.
If the mixture deviates from Raoult’s Law, then azeotropes are formed, and for azeotropes, bubble points and dew points are the same. Mixtures of non-azeotropic liquids under any circumstances are referred to as azeotropic.
Azeotrope Mixture Types
There are different types of azeotropic mixtures, and they are as follows:
1. Minimum Boiling Azeotropes or Negative Azeotrope
Azeotropic mixtures with a higher boiling point in their constitutions are maximum boiling azeotropes. Water boils at 373 K, and hydrochloric acid boil at about 188 K, while azeotropes boil at around 383 K, which is a boiling point greater than its constituents. Consider, for example, hydrochloric acid consisting of a weight concentration of approximately twenty per cent and 79 per cent of water.
Examples:
- Separation of water and isobutanol.
- Dehydration of ethanol.
- Separation of cyclohexane and benzene.
2. Maximum Boiling Azeotropes or Positive Azeotrope
Similarly, an azeotropic mixture that has a boiling point lesser than its constituents is known as a minimum boiling azeotrope. Consider, for example, ethanol consisting of a weight concentration of approximately ninety-five per cent and four per cent of water. Water boils at 373 K, and ethanol boils at about 351.5 K, while azeotropes boil at around 351.15 K, suggesting a boiling point lower than its constituents.
3. Heterogeneous and Homogeneous Azeotropes
When azeotropes are present in mixture constitutions and are not fully miscible, they are called heterogeneous azeotropes. On the other hand, homogeneous azeotropes are azeotropes where a mixture’s constitutions are completely miscible. Hetero Azeotropic distillation normally consists of two phases of a liquid.
4. Azeotropes Based on the Number of Constituents
Binary azeotropes are two-constituted azeotropes. Ternary azeotropes are more than three azeotropic constitutions.
Azeotrope Examples
Boiling a 95% solution of ethanol in water will produce a 95% ethanol vapour. It is not necessary to use distillation to obtain higher ethanol concentrations. Alcohol and water are miscible, making it possible to combine any quantity of ethanol with any quantity of water to produce a homogeneous solution that works like an azeotrope.
On the other side, chloroform and water make up a heteroazeotrope. These two liquids are separated by a mix that comes mainly from the top layer of the water, with a small amount of chloroform being dissolved and a small amount of water dissolved from the chloroform base layer. The mixture can be streamed at a lower temperature when the two layers are heated together as the boiling water or chloroform. The resultant vapour will be 97% chloroform and 3% water, irrespective of the liquid ratio. The condensation of vapours contributes to fixed layers of composition. The upper layer comprises 4.4 per cent of thickness, while the lower condensate layer contains 95.6 per cent.
Azeotrope Separation
We do not use fractional distillation to isolate an azeotrope component. There are different types of distillation methods that are used. They are as follows:
- Entrainer distillation: Here, there is an application of an entrainer, which is a material that changes the stability of one of the azeotrope components. And a heterogeneous azeotrope IS is formed. The distillation is also termed azeotropic distillation while using a simulator.
- Pressure swing distillation: In this process, the pressure is changed in order to modify the mixture composition and enrich the distillate with the desired portion.
- Pervaporation: This is a process of separating materials with the help of a membrane that will be permeable to only one material compared to the other.
Azeotropic Distillation
Azeotropic distillation in today’s processes is an integral unit activity. In the chemical process industry (CPI), chemical specialities and the food industry, applications of azeotropic distillation can be easily seen. The main advantages of azeotropic distillation are to allow the separation and re-energisation of chemicals which cannot be effectively separated by conventional distillation systems such as the azeotropic or pinch point systems. The key drawbacks of azeotropic distillation are the overall column diameter required to increase the volume of steam and the sophistication of the controls in comparison with the simple distillation of the azeotropic agent.
A minimum-boiling azeotrope may be produced by adding a compound forming an azeotrope (trainer), which cannot be differentiated by the conventional distillation in an established azeotropic or closely boiling mixture. The oxidation of alcohol is an example. A minimum boiling azeotrope is produced by ethanol, which means that ethanol cannot fully be dehydrated by modern distillation. Benzene produces ethanol and a water ternary azeotrope that boils at a lower temperature to remove the surface water (with ethanol) and so leaves dry ethanol to the floor.
In some situations, as in the processing of esters, an azeotrope that occurs within the system can be beneficially used to purify a compound. Alcohol esterification is a reversible reaction. The reaction will not be completed in the presence of the product as the equilibrium is reduced. If one of the components is extracted (in this case, water) using water/alcohol azeotrope (nearly all alcohols form azeotropes with water from C2 to C20), the reaction is guided in favour of the ester component.
Relative volatility reflects a difference between the mixture of variable volatility. When the vapour and liquid phase compositions are the same, the factor volatility is the same, and the relative volatility is the same as 1. The further away from 1 is the relative volatility, the simpler it is to distinguish the mixture components.
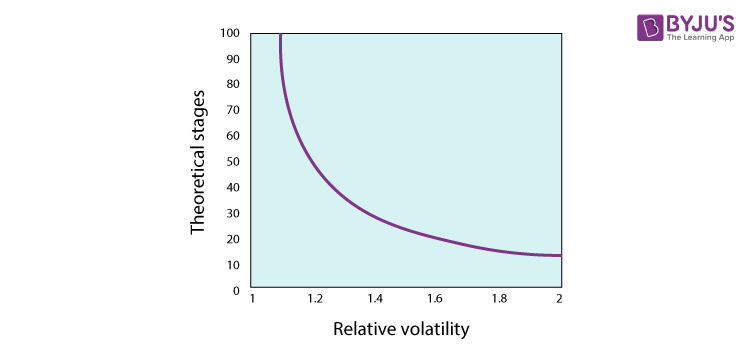
As we can see in the figure, infinity approaches the number of trays needed asymptotically, and separation becomes impossible as unification approaches relative volatility. The existence of a pinch point contributes to relative unity uncertainty. Due to numerous separation phases, pinch-forming components are technically feasible but often not economical.
The vapour is mixed in a single liquid phase with homogeneous azeotropes. The trainer should be recovered through further fractionation or extraction in a homogeneous azeotropic environment.
The vapour is in harmony with the two phases of air. There are heterogeneous azeotropes. Heterogeneous azeotropic distillation is widely used for insulation of azeotropic or closed boiling mixtures by producing a minimum boiling azeotropic and reclaiming the equipment by using fluid immiscibility.
Azeotropes Distillation Key Terms
- The process in which the new component or entrainer is added to the mixture to form a lower boiling azeotrope of the heterogeneous solution with one or more of the feed components.
- Azoetropes are either removed as a distillate or at the bottom.
- Distillation helps to separate two or more azeotropes that are mixed together.
Azeotropic Mixtures – Ideal and Non-Ideal Solutions


Comments