Biomolecules JEE notes PDF helps students to have a quick revision before the entrance exam. We provide notes in order to help students understand each topic in a better way. Biomolecules JEE notes PDF includes all the important definitions, facts and formulas from the topic biomolecules. Students are recommended to go through these notes so that they won’t miss any important points for the exam.
Click here to download the PDF:- Download Biomolecules JEE Notes PDF
The topics covered in this article include characteristics of carbohydrates, proteins and nucleic acids and hormones, the classification of carbohydrates and vitamins on the basis of their structures, the difference between DNA and RNA, and the role of biomolecules in the biosystem. Biomolecules are the organic compounds which form the basis of living organisms. They build up the living system and are responsible for its growth and maintenance.
Table of Contents
- Carbohydrates
- Preparation of Glucose
- Proteins
- Vitamins
- Solved Examples
- Practice Problems
- Frequently Asked Questions
Carbohydrates
Carbohydrates are defined as optically active polyhydroxy aldehydes or ketones or the compounds which produce such units on hydrolysis. Carbohydrates are also known as saccharides. According to their behaviour on hydrolysis, carbohydrates are classified into three groups: Monosaccharides, Oligosaccharides and Polysaccharides.
Monosaccharides are the simplest carbohydrates. They cannot be hydrolysed into simpler compounds. For example, glucose and ribose. Oligosaccharides are carbohydrates which give 2 to 10 monosaccharide units on hydrolysis. For example, sucrose, lactose and maltose. They are further classified as disaccharides, trisaccharides, tetrasaccharides, etc. Polysaccharides are carbohydrates which give a large number of monosaccharide units on hydrolysis. For example, cellulose and starch. Polysaccharides are not sweet in taste, and hence they are also called non-sugars.
Monosaccharides
On the basis of the number of carbon atoms and the functional group, monosaccharides are again classified as ketose and aldose. If it contains a keto group, it is called a ketose. If a monosaccharide contains an aldehyde group, it is called an aldose.
Preparation of Glucose
Glucose is the monomer for many other carbohydrates. It is probably the most abundant organic compound on the earth. Glucose occurs freely in nature as well as in the combined form. It is present in sweet fruits and honey. The molecular formula of glucose is C6H12O6.
a) From sucrose
C12H22O11 + H2O → C6H12O6 + C6H12O6
Sucrose Glucose Fructose
b) From starch
(C6H10O5)n + nH2O → nC6H12O6
Starch or cellulose Glucose
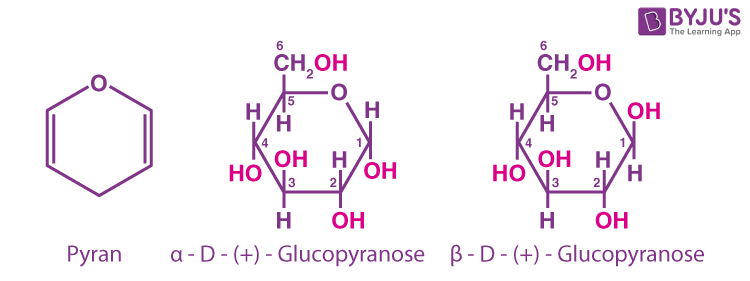
The cyclic structure of glucose is shown below.
Fructose
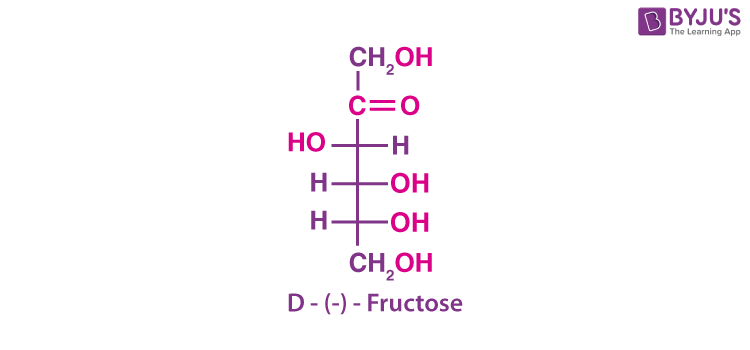
Fructose is obtained along with glucose by the hydrolysis of the disaccharide, namely sucrose. It is found in fruits, honey and vegetables. It is a natural monosaccharide. It is used as a sweetener in its pure form. It is an important ketohexose. Fructose has molecular formula C6H12O6. It belongs to the D-series and is a laevorotatory compound. It is appropriately written as D-(–)-fructose. The open-chain structure of fructose is shown below.
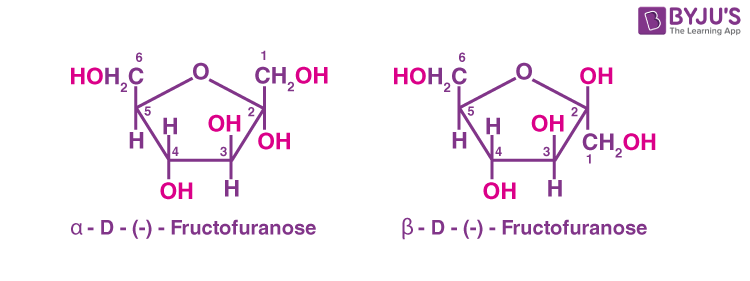
The cyclic structures of two anomers of fructose (Haworth structures) are given below.
Disaccharides
Disaccharides yield two molecules of either the same or different monosaccharides on hydrolysis with dilute acids or enzymes. The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage through an oxygen atom is called a glycosidic linkage.
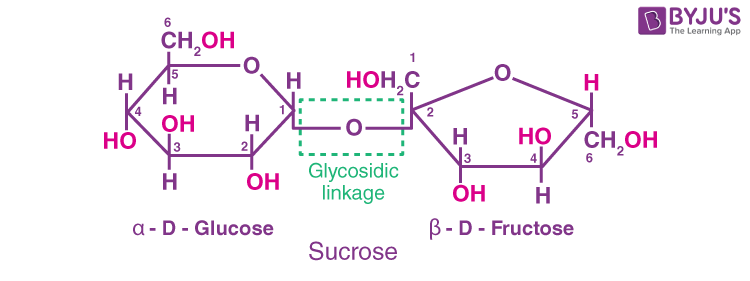
(a) Sucrose: It is one of the common disaccharides which, on hydrolysis, gives an equimolar mixture of D-(+)-glucose and D-(-) fructose.
C12H22O11+H2O → C6H12O6 + C6H12O6
By a glycosidic linkage between C1 of α-D-glucose and C2 of β-D-fructose, these two monosaccharides are held together.
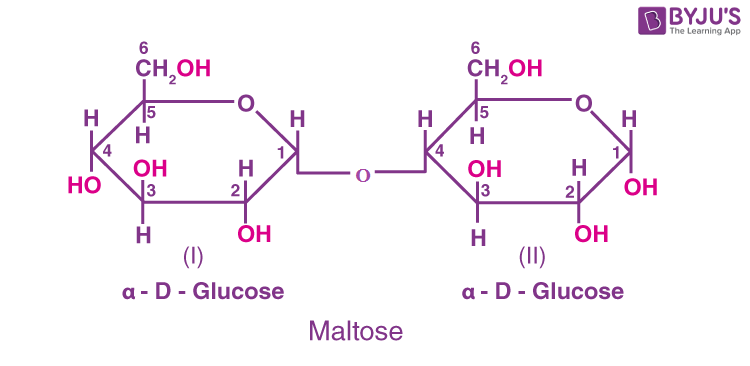
(b) Maltose: It is composed of two α-D-glucose units in which C1 of one glucose (I) is linked to C4 of another glucose unit (II). The free aldehyde group can be produced at C1 of the second glucose in solution. It exhibits reducing properties, so it is a reducing sugar.
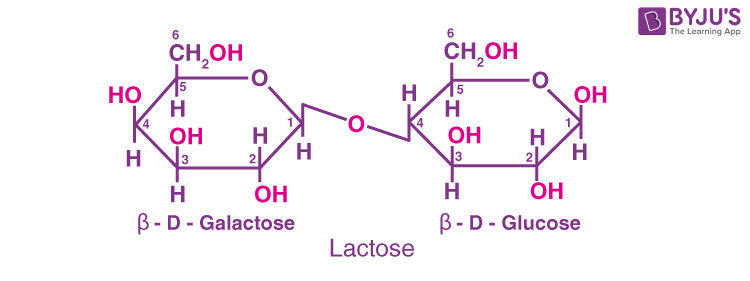
(c) Lactose: It is also a reducing sugar. This disaccharide is found in milk, hence it is known as milk sugar. It is composed of β-D-galactose and β-D-glucose. The linkage is between C1 of galactose and C4 of glucose.
Polysaccharides
A large number of monosaccharide units are joined together by glycosidic linkages to form polysaccharides. They mainly act as food storage or structural materials.
(i) Starch: It is the main storage polysaccharide of plants and a dietary source for human beings. High content of starch is found in cereals, roots, tubers and some vegetables. It is a polymer of α-glucose and consists of Amylose and Amylopectin components. Amylose is water soluble, and Amylopectin is insoluble in water.
(ii) Cellulose: Cellulose occurs in plants, and it is the most abundant organic substance in the plant kingdom.
(iii) Glycogen: The carbohydrates are stored in the animal body as glycogen. Glycogen is also called animal starch.
Proteins
All proteins are polymers of α-amino acids. The main sources of proteins are milk, cheese, fish, meat, pulses, peanuts, etc. They form the fundamental basis of the structure and functions of life. They are also required for the growth and maintenance of the body.
Amino acids
Amino acids contain carboxyl (-COOH) and amino (-NH2 ) functional groups. According to the relative position of the amino group with respect to the carboxyl group, it can be classified as α, β, γ, δ and so on. On hydrolysis of proteins, only α-amino acids are obtained.
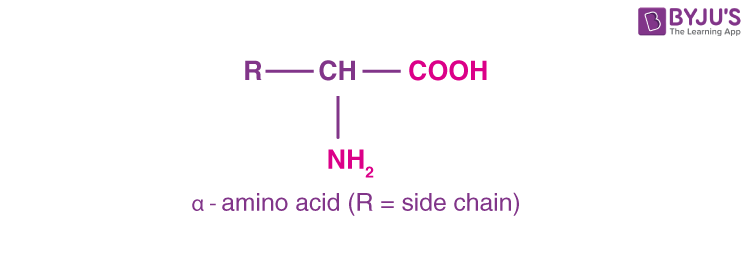
Essential and non-essential amino acids
Non-essential amino acids are amino acids which can be synthesised in the body. The amino acids which cannot be synthesised by the body are called essential amino acids. They must be obtained through diet.
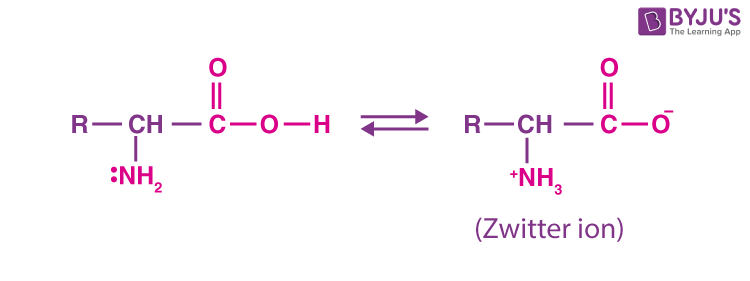
Zwitterion
In an aqueous solution, the carboxyl group can lose a proton, and an amino group can accept a proton, giving rise to a dipolar ion known as the Zwitterion.
On the basis of their molecular shape, proteins can be classified into fibrous proteins and globular proteins. Keratin and myosin are examples of fibrous proteins. They are insoluble in water. Insulin and albumin are examples of globular proteins. They are soluble in water.
The structure and shape of proteins can be studied at four different levels.
Primary structure: Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence, and it is this sequence of amino acids that is said to be the primary structure of that protein.
Secondary structure: The secondary structure of a protein refers to the shape in which a long polypeptide chain can exist.
Tertiary structure: The tertiary structure of proteins represents the overall folding of the polypeptide chains.
Quaternary structure: Some proteins are made up of two or more polypeptide chains known as sub-units. The spatial arrangement of these subunits with respect to each other is known as quaternary structure.
Denaturation of proteins
The secondary or tertiary structure of proteins gets disturbed by changes in pH or temperature, and they are not able to perform their functions. This is called the denaturation of proteins. The coagulation of egg white on boiling and curdling of milk are examples.
Importance of proteins
a) Hormones, such as insulin and glucagon, are proteins.
b) Proteins participate in the growth and repair mechanism of body tissues.
c) A protein called fibrinogen helps to stop bleeding.
d) Oxygen is transported to different tissues from the blood by haemoglobin which is a protein.
e) Proteins are structural components of cells.
f) The proteins known as immunoglobulins defend against infections.
Enzymes
Various chemical reactions in living organisms involve a sequence of reactions, and all these reactions occur in the body under very mild conditions. This occurs with the help of certain biocatalysts called enzymes. Maltase is the enzyme that catalyses the hydrolysis of maltose into glucose.
Vitamins
These are organic compounds required in the diet in small amounts to perform specific biological functions for normal maintenance of optimum growth and health of the organism.
a) Fat-soluble vitamins: These vitamins are soluble in fat and oils but insoluble in water and are kept in this group. They are vitamins A, D, E and K.
b) Water-soluble vitamins: B group vitamins and vitamin C are soluble in water, and thus they are grouped together. Water-soluble vitamins are readily excreted in urine and cannot be stored (except vitamin B12) in the body. So, these vitamins must be supplied regularly in the diet.
The following table shows vitamins, their deficiency diseases and their sources.
| Vitamins | Deficiency Diseases | Sources |
| Vitamin A | Xerophthalmia, Night blindness | Fish liver oil, carrots, butter and milk |
| Vitamin B1 (Thiamine) | Beriberi | Green vegetables, cereals, yeast, and milk. |
| Vitamin B2 (Riboflavin) | Digestive disorders and burning sensation of the skin, cheilosis | Milk, eggwhite, liver, kidney |
| Vitamin B6 (Pyridoxine) | Convulsions | Yeast, milk, egg yolk, cereals and grams |
| Vitamin B12 | Pernicious anaemia | Meat, fish, egg and curd |
| Vitamin C (Ascorbic acid) | Scurvy (bleeding gums) | Citrus fruits, amla and green leafy vegetables |
| Vitamin D | Rickets and osteomalacia | Exposure to sunlight, fish and egg yolk |
| Vitamin E | Increased fragility of RBCs and muscular weakness | Vegetable oils like wheat germ oil, sunflower oil, etc. |
| Vitamin K | Increased blood clotting time | Green leafy vegetables |
Nucleic acids
The particles in the nucleus of the cell, responsible for heredity, are called chromosomes which are made up of proteins and another type of biomolecule called nucleic acids. The two types of nucleic acids are DNA and RNA. In DNA molecules, the sugar is β-D-2-deoxyribose. In RNA molecules, it is β-D-ribose.
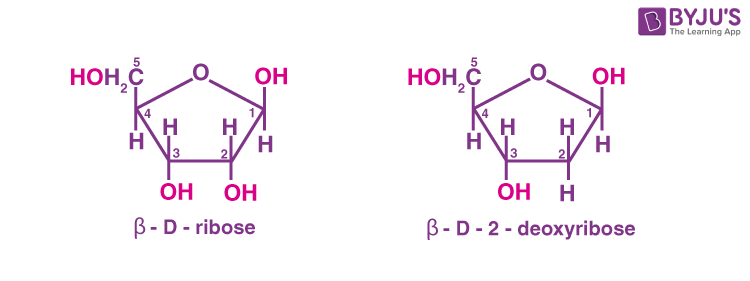
DNA contains four bases.
- Adenine (A)
- Guanine (G)
- Cytosine (C)
- Thymine (T)
RNA also contains four bases, the first three bases are the same as in DNA, but the fourth one is uracil (U). There are three types of RNA, and they are mRNA, rRNA and tRNA, which carry out protein synthesis in the cell.
| DNA | RNA |
| DNA is a double-stranded molecule. | RNA is a single-stranded molecule. |
| DNA is the chemical basis of heredity. | RNA helps in protein synthesis. |
| Adenine (A), Guanine (G), Cytosine (C)
Thymine (T) is the 4 bases. |
Adenine (A), Guanine (G), Cytosine (C)
Uracil (U) is the 4 bases. |
| DNA contains deoxyribose. | RNA contains ribose as sugar. |
Hormones
Hormones are produced by endocrine glands in the body and are poured directly into the blood, which transports them to the site of action. They act as intercellular messengers and also help to maintain the balance of biological activities in the body.
Solved Examples
Question 1:
How is glucose related to fructose?
1) Functional group isomerism
2) Rotamers
3) Position isomerism
4) Geometrical isomerism
Solution:
Glucose and fructose have the same molecular formula, C6H12O6, but differ in the functional group having aldehydic and ketonic groups, respectively. So, glucose and fructose are the functional group isomers.
Hence, option 1 is the answer.
Question 2:
The sweetest carbohydrate is _____.
1) sucrose
2) glucose
3) fructose
4) lactose
Solution:
Fructose is the sweetest of all carbohydrates that occur naturally.
Hence, option 3 is the answer.
Question 3:
A carbohydrate is treated with α-naphthol and conc. H2SO4. What colour will be formed at the junction of two liquids?
1) Blood-red
2) Violet
3) Brown
4) Orange
Solution:
Carbohydrate reacts with α-naphthol and concentrated H2SO4 to give violet colour. This is Molisch’s test for carbohydrates.
Hence, option 2 is the answer.
Question 4:
Milk changes after digestion into ______.
1) cellulose
2) fructose
3) glucose
4) lactose
Solution:
The main carbohydrate in milk is lactose. Before absorption, lactose must be broken down into its two individual sugars (glucose and galactose). Lactose is broken down by the enzyme lactase that is secreted by the intestinal cells. So, the milk changes into glucose and galactose after digestion.
Hence, option 3 is the answer.
Question 5:
Cellulose is a polymer of ______.
1) glucose
2) fructose
3) ribose
4) sucrose
Solution:
Cellulose is an organic compound with the formula (C6H10O5)n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→ 4) linked D glucose units.
Hence, option 1 is the answer.
Practice Problems
1. Glucose gives silver mirror with ammoniacal silver nitrate because it has _____.
1) aldehyde group
2) ester group
3) ketone group
4) alcoholic silver nitrate
2. Lactose gives_____ on hydrolysis.
1) glucose
2) glucose and galactose
3) fructose
4) glucose and fructose
3. The two forms of D-glucopyranose obtained from the solution of D-glucose are called _____.
1) isomers
2) anomers
3) epimers
4) enantiomers
4. Water insoluble component of starch is _____.
1) amylopectin
2) amylose
3) cellulose
4) None of these
5. Which of the following enzymes hydrolysis starch to glucose?
1) Amylase
2) Invertase
3) Lactase
4) Maltase
Video Lessons
Biomolecules and Polymers

Peptide Bonds

Biomolecules – Top 10 Most Important and Expected JEE Questions

Frequently Asked Questions
What are monosaccharides?
The simplest carbohydrates which cannot be hydrolysed into smaller molecules are called monosaccharides. Their general formula is (CH2O)n, where n = 3 – 7
For example, glucose, fructose etc.
Why cannot vitamin C be stored in our body?
Vitamin C cannot be stored in our body because it is a water-soluble vitamin. It gets excreted from the body through sweat or urine. So, they must be supplied in the diet regularly.
What are the expected products of the hydrolysis of lactose?
On hydrolysis, lactose gives β-D-galactose and β-D-glucose.
What is a glycosidic linkage?
The two monosaccharide units are joined together through an oxide linkage formed by the loss of a molecule of water. Such a linkage between two monosaccharide units through an oxygen atom is called glycosidic linkage.
Where does the water present in the egg go after boiling the egg?
A process that changes the physical and biological properties of proteins without affecting the chemical composition of proteins is called the denaturation of proteins. In an egg, denaturation of protein is the coagulation of albumin present in the white of an egg. When an egg is boiled, the globular proteins (albumin of egg white) coagulate (denature) to a rubber-like insoluble mass. This insoluble mass absorbs all water present in the egg as it makes hydrogen bonds with it.
Comments