Contact process is one of the most popular or common methods to manufacture sulphuric acid. The contact process was invented by a British merchant named Peregrine Phillips. It was patented in the year 1831. Apart from being an economical process for manufacturing sulphuric acid, sulphur trioxide and oleum are also obtained from this process. We will be learning more about the contact process in this lesson.
Download Complete Chapter Notes of The p- Block Elements (Group 15 – 18)
Download Now
Sulphuric Acid (H2SO4)
It is said that, if one could estimate the amount of sulphuric acid produced in a country, then that information is more than enough to estimate the industrial growth of that particular country. This is because sulphuric acid is an essential raw material for almost everything that is industrially made. It is commonly used in fertiliser manufacturing, oil refining, mineral processing and it is even used in wastewater processing. Some other uses include domestic acidic drain cleaners, as electrolytes in lead-acid batteries, as a dehydrating agent, etc.
In a nutshell, sulphuric acid is a strong mineral acid characterised by its strong dehydrating and oxidising nature. It has a chemical formula H2SO4. This acid is colourless with a pungent smell. It is soluble in water and releases heat on contact. On top of that, this acid is corrosive to metals and most other organic matters like tissue, wood, etc. On contact with such organic substances, sulphuric acid instantly dehydrates them causing char to form. Sulphuric acid has a density of 1.83 g / cm3.
The molecular structure of sulphuric acid is represented below in the form of a line-wedge-dash structure.
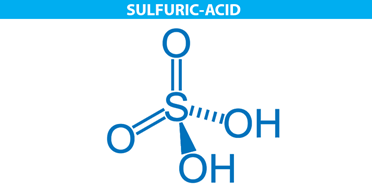
Properties of sulphuric acid are listed below:
| Chemical formula | H2SO4 |
| Molar mass | 98.079 g/mol |
| Appearance | Clear, colourless liquid |
| Density | 1.8302 g/cm3 |
| Melting point and boiling point | 50.560 F and 6390F |
| Solubility in water | Exothermic, miscible |
| Viscosity | 26.7 cP |
Manufacture of Sulphuric Acid By Contact Process
Generally, there are several ways to manufacture sulphuric acid. Each of them varies in the effort, cost, and purity of the sulphuric acid that is produced. However, the most common process among these is the contact process. Let us discuss this process in detail below.
The manufacture of sulphuric acid using the contact process involves four steps. These include;
- Extraction of sulphur.
- Preparation of sulphur dioxide.
- Conversion of sulphur dioxide to sulphur trioxide.
- Conversion of sulphur trioxide to sulphuric acid.
Extraction of Sulphur
Pure sulphur is required for the production of sulphur dioxide gas. There are many sources for extracting sulphur. The most important one among these is the recovery from natural gas and oil. The organic or mineral parts of these are removed to obtain sulphur.
Apart from the extraction of pure sulphur, there are few ways by which sulphur dioxide can be extracted. Metal refining is one of them. Many metal ores occur as sulphides in the soil. Later they are roasted to obtain their respective oxide and sulphur dioxide.
Consider the extraction process of lead:
2PbS + 3O2 → 2PbO + 2SO2
Likewise, sulphur dioxide can be obtained from the metallurgy of copper, nickel, zinc, etc. In China, most of the sulphur dioxide is extracted from pyrite, an iron sulphide ore.
Preparation of Sulphur Dioxide
Sulphur dioxide can be prepared by burning pure sulphur in the presence of excess air. This causes the formation of dioxide of sulphur. The process of sulphur dioxide preparation is as follows:
- Molten sulphur is pumped into the stationary atomizer. This causes the formation of atomized sulphur. This atomized sulphur is applied to the hot furnace. Air is preheated and dried using a sulphuric acid dehydrator and is also applied to the hot furnace.
- Air and atomized molten sulphur are kept at one end of the hot furnace. When a reaction occurs sulphur dioxide is produced at the other end.
Also Read: Oxides of Sulphur
The chemical reaction involved in this process is shown below:
- Melting and atomization.
- Production of sulphur dioxide using the hot furnace.
S(g) + O2 → SO2(g) + Δ
Conversion of Sulphur Dioxide to Sulphur Trioxide
This is a really delicate step in the contact process. This is partly because it produces corrosive sulphur trioxide. Also, this process is a reversible reaction, and hence involves setting different reaction parameters to obtain maximum output. The reaction equation is represented below:
2SO2(g) + O2(g) ⇌ SO3 (g) ΔH = -196 KJ mol-1
The detailed steps involved are pictured below:
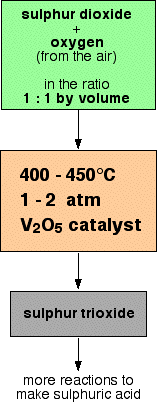
Sulphur dioxide and oxygen are combined at a ratio of 1:1 inside a lead chamber. The temperature is set to 400 – 450 degrees Celsius with 1 to 2 atm. The process is catalysed by the vanadium pentoxide catalyst.
Conversion of Sulphur Trioxide to Sulphuric Acid
Dilution of sulphur trioxide in water can yield sulphuric acid. This is, however, really dangerous and hence not followed. The addition of sulphur trioxide to water is highly exothermic and causes the fuming of sulphuric acid. The fumes prevent the further dissolution of sulphur trioxide in water.
The accepted method is to dilute the sulphur trioxide in sulphuric acid. This produces oleum.
SO3 (g) + H2SO4 → H2S2O7 (1)
Oleum can be further diluted in water to obtain concentrated sulphuric acid.
H2S2O7 (1) + H2O(1) → 2H2SO4(1)
These are the steps involved in producing sulphuric acid via the contact process.
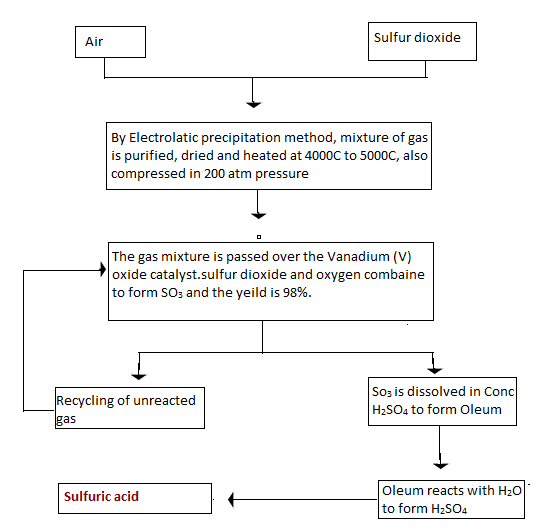
The preparation of sulphuric acid by the contact process can be explained in a single diagram as shown below
Essential Conditions for Obtaining Maximum Sulphuric Acid Yield
Concentration Consideration
A 1:1 proportion of sulphur dioxide and oxygen is used. From the equation, we can see that there is always an excess in oxygen if we use this ratio. According to Le’ Chatelier’s principle, if the amount of reactant becomes high, the equilibrium will tip towards the product side. Since the amount of oxygen is always higher in the reactant side, the reaction will yield sulphur trioxide.
Temperature Consideration
This reaction is exothermic in nature as shown:
2SO2(g) + O2(g) ⇌ SO3 (g) ΔH = -196 KJ mol-1
Hence, according to Le’ Chateliers’ principle, a lower temperature yields more sulphur trioxide. However, it is not a feasible way to reduce the temperature too low. Some heat is always required for keeping the rate of reaction to the required level. Hence, most often an optimum temperature of 400 – 450 degrees celsius is selected.
Pressure Consideration
From the equation, we can see that on the reactant side, there are three reactant molecules and on the product side only two. That means the application of excess pressure favours the production of more sulphur trioxide. That is, the equilibrium will tip towards the product side.
According to the Le Chataliers’ principle, the excess pressure causes the system to yield more products so that finally the pressure can subside by reducing the number of molecules from three to two. However, a low value of pressure generally 1 – 2 atm is preferred due to safety and economic concerns.
The Catalyst
The catalyst used in the contact process is vanadium pentoxide. The action of the catalyst is not to yield more product but to make the reaction faster by forcing the reaction to reach equilibrium quickly. Nonetheless, the plant is designed in a manner to not let the reaction stay in equilibrium.
Summary
- The contact process is a present method of producing concentrated sulphuric acid which is required for industrial use.
- The catalyst used during the process is vanadium oxide.
- The contact process is divided into three stages:
1 Stage: Sulphur dioxide preparation and purification.
2 stage: Conversion of sulphur dioxide to sulphur trioxide by catalytic oxidation.
3 Stage: Sulphur trioxide is converted into Sulphuric acid.

Comments