Corrosion is one of the most common phenomena that we observe in our daily lives. You must have noticed that some objects made of iron are covered with an orange or reddish-brown coloured layer at some point in time. The formation of this layer is the result of a chemical process known as rusting, which is a form of corrosion.
Download Complete Chapter Notes of Electrochemistry
Download Now
Cossorion, in general, is a process through which refined metals are converted into more stable compounds such as metal oxides, metal sulfides, or metal hydroxides. Likewise, the rusting of iron involves the formation of iron oxides via the action of atmospheric moisture and oxygen. If we look at the science behind corrosion, then we can say that it is a spontaneous/irreversible process wherein the metals turn into a much more stable chemical compound like oxides, sulphides, hydroxides, etc. We will delve deeper into the concept of corrosion and understand its different factors, including its meaning, types, prevention and more in this lesson.
Table of Contents
- Corrosion Definition
- Factors Affecting Corrosion
- Types of Corrosion
- Corrosion Examples and Reactions
- Prevention of Corrosion
Corrosion Definition
What is Corrosion? It is basically defined as a natural process that causes the transformation of pure metals into undesirable substances when they react with substances like water or air. This reaction causes damage and disintegration of the metal, starting from the portion of the metal exposed to the environment and spreading to the entire bulk of the metal.
Corrosion is usually an undesirable phenomenon since it negatively affects the desirable properties of the metal. For example, iron is known to have good tensile strength and rigidity (especially alloyed with a few other elements). However, when subjected to rusting, iron objects become brittle, flaky, and structurally unsound. On the other hand, corrosion is a diffusion-controlled process, and it mostly occurs on exposed surfaces. Therefore, in some cases, attempts are made to reduce the activity of the exposed surface and increase a material’s corrosion resistance. Processes such as passivation and chromate conversion are used for this purpose. However, some corrosion mechanisms are not always visible, and they are even less predictable.
On the other hand, corrosion can be classified as an electrochemical process since it usually involves redox reactions between the metal and certain atmospheric agents such as water, oxygen, sulphur dioxide, etc.
Do All Metals Corrode?
Metals placed higher in the reactivity series, such as iron, zinc, etc., get corroded very easily, and metals placed lower in the reactivity series, like gold, platinum and palladium, do not corrode. The explanation lies in the fact that corrosion involves the oxidation of metals. As we go down, the reactivity series tendency to get oxidised is very low (oxidation potentials are very low).
Also read: Oxidation and Reduction
Interestingly, aluminium doesn’t corrode, unlike other metals, even though it is reactive. This is because aluminium is covered by a layer of aluminium oxide already. This layer of aluminium oxide protects it from further corrosion.
Factors Affecting Corrosion
1. Exposure of the metals to air containing gases like CO2, SO2, SO3 etc.
2. Exposure of metals to moisture, especially salt water (which increases the rate of corrosion).
3. Presence of impurities like salt (For example, NaCl).
4. Temperature: An increase in temperature increases corrosion.
5. Nature of the first layer of oxide formed: Some oxides like Al2O3 form an insoluble protecting layer that can prevent further corrosion. Others, like rust, easily crumble and expose the rest of the metal.
6. Presence of acid in the atmosphere: Acids can easily accelerate the process of corrosion.
Rate of Corrosion
The Deal–Grove model is often used to describe the formation of an oxide layer. This model helps in predicting and controlling oxide layer formation in a lot of diverse situations. Apart from this, the weight loss method is also used to measure corrosion. In this method, a clean, weighed piece of the metal or alloy is exposed to the corrosive environment for a certain duration. This is followed by a cleaning process that removes the corrosion products. The piece is then weighed to determine the loss of weight.
The rate of corrosion (R) is calculated as:
Where,
k = constant,
W = weight loss of the metal in time t,
A = surface area of the metal exposed,
ρ is the density of the metal (in g/cm³).
Types of Corrosion
Some of the corrosion types include the following:
(i) Crevice Corrosion
Whenever there is a difference in ionic concentration between any two local areas of a metal, a localised form of corrosion known as crevice corrosion can occur. For instance, this form of corrosion mostly occurs in confined spaces (crevices). Examples of areas where crevice corrosion can occur are gaskets, the undersurface of washers, and bolt heads. All grades of aluminium alloys and stainless steels also undergo crevice corrosion. This is mainly because of the formation of a differential aeration cell that leads to the formation of corrosion inside the crevices.
(ii) Stress Corrosion Cracking
Stress corrosion cracking can be abbreviated to ‘SCC’ and refers to the cracking of the metal as a result of the corrosive environment and the tensile stress placed on the metal. It often occurs at high temperatures.
For example, stress corrosion cracking of austenitic stainless steel in chloride solution.
(iii) Intergranular Corrosion
Intergranular corrosion occurs due to the presence of impurities in the grain boundaries that separate the grain formed during the solidification of the metal alloy. It can also occur via the depletion or enrichment of the alloy at these grain boundaries.
For example, Aluminum-base alloys are affected by IGC.
(iv) Galvanic Corrosion
When there exists an electric contact between two metals that are electrochemically dissimilar and are in an electrolytic environment, galvanic corrosion can arise. It refers to the degradation of one of these metals at a joint or at a junction. A good example of this type of corrosion would be the degradation that occurs when copper, in a salt-water environment, comes in contact with steel.
For example, when aluminium and carbon steel are connected and immersed in seawater, aluminium corrodes faster, and steel is protected.
(iv) Pitting Corrosion
Pitting Corrosion is very unpredictable and, therefore, is difficult to detect. It is considered one of the most dangerous types of corrosion. It occurs at a local point and proceeds with the formation of a corrosion cell surrounded by the normal metallic surface. Once this ‘pit’ is formed, it continues to grow and can take various shapes. The pit slowly penetrates metal from the surface in a vertical direction, eventually leading to structural failure if left unchecked.
For example, consider a droplet of water on a steel surface, pitting will initiate at the centre of the water droplet (anodic site).
(v) Uniform Corrosion
This is considered the most common form of corrosion wherein an attack on the surface of the metal is executed by the atmosphere. The extent of the corrosion is easily discernible. This type of corrosion has a relatively low impact on the performance of the material.
For example, a piece of zinc and steel immersed in diluted sulphuric acid would usually dissolve over its entire surface at a constant rate.
(vi) Hydrogen Grooving
This is a corrosion of the piping by grooves that are formed due to the interaction of a corrosive agent, corroded pipe constituents, and hydrogen gas bubbles. The bubbles usually remove the protective coating once it comes in contact with the material.
(vii) Metal Dusting
Metal dusting is a damaging form of corrosion that occurs when vulnerable materials are exposed to certain environments with high carbon activities, including synthesis gas. The corrosion results in the break-up of bulk metal to metal powder. Corrosion occurs as a graphite layer is deposited on the surface of the metals from carbon monoxide (CO) in the vapour phase. This graphite layer then goes on to form meta-stable M3C species (where M is a metal) that usually move away from the metal surface. In some cases, no M3C species may be observed. This means that the metal atoms have been directly transferred into the graphite layer.
(viii) Microbial Corrosion
Microbial corrosion, which is also known as microbiologically influenced corrosion (MIC), is a type of corrosion that is caused by microorganisms. The most common one is chemoautotrophs. Both metallic and non-metallic materials, either in the presence or absence of oxygen, can be affected by this corrosion.
(viii) High-temperature Corrosion
High-temperature corrosion, as the name suggests, is a type of corrosion of materials (mostly metals) due to heating. Chemical deterioration of metal can occur due to a hot atmosphere that contains gases such as oxygen, sulphur, or other compounds. These compounds are capable of oxidising the materials (metals in this case) easily. For example, materials used in car engines have to resist sustained periods at high temperatures, during which they can be affected by an atmosphere containing corrosive products of combustion.
Corrosion Examples, Reactions and Effects
Here are some typical examples of corrosion, as seen mostly in metals.
1. Copper Corrosion
When copper metal is exposed to the environment, it reacts with the oxygen in the atmosphere to form copper (I) oxide, which is red in colour.
2Cu(s) + ½ O2(g) → Cu2O(s)
Cu2O further gets oxidised to form CuO, which is black in colour.
Cu2O(s) + ½ O2(g) → 2CuO(s)
This CuO reacts with CO2, SO3 and H2O (present in the atmosphere to form Cu2(OH)2(s) (Malachite), which is blue in colour and Cu4SO4(OH)6(s) (Brochantite), which is green in colour.
This is why we observe copper turning bluish-green in colour.
A typical example of this is the colour of the Statue of Liberty, which has the copper coating on it turning blue-green in colour.
2. Silver Tarnishing
Silver reacts with sulphur and sulphur compounds in the air, giving silver sulphide (Ag2S), which is black in colour. Exposed silver forms Ag2S as it reacts with the H2S(g) in the atmosphere, which is present due to certain industrial processes.
2Ag(s) + H2S(g) → Ag2S(s) + H+2+(g)
3. Corrosion of Iron (Rusting)
Rusting of iron, which is the most commonly seen example, happens when iron comes in contact with air or water. The reaction could be seen as a typical electrochemical cell reaction. Consider the diagram given below.
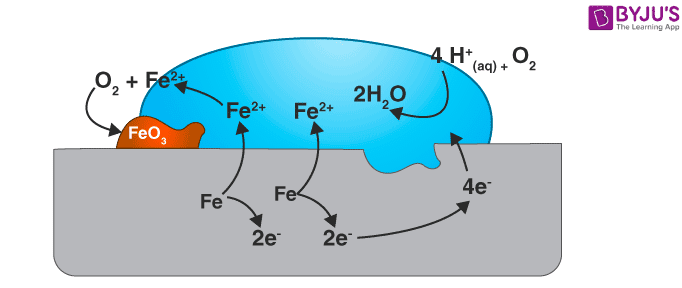
Here, metal iron loses electrons and gets converted to Fe{aq}2+ (this could be considered as the anode position). The electrons lost will move to the other side, where they combine with H+ ions. H+ ions are released either by H2O or by H2CO3 present in the atmosphere (this could be considered as the cathode position).
The Hydrogen, thus formed by the reaction of H+ and electrons, react with oxygen to form H2O.
Anode reaction
2Fe(s) → 2Fe2+ + 4e– ;
Cathode reaction
Overall reaction
2Fe(s) + O2(g) + 4H+(aq) → 2Fe2+(aq) + 2H2O(l)Eocell = 1.67V
The Fe2+ ions formed at the anode react with oxygen in the atmosphere, thereby getting oxidised to Fe3+ and forming Fe2O3, which comes out in the hydrated form as Fe2O3.xH2O
Fe2+ + 3O2 → 2Fe2O3
Fe2O3 + xH2O → Fe2O3. xH2O (rust)
Also Read: Rusting of Iron and Prevention
Other examples include,
- Corrosion of Zinc when it reacts with oxygen and HCl to form white-coloured ZnCl2.
- Corrosion of Tin to form black-coloured Na2[Sn(OH)2].
Effects
Corrosion can have a varying degree of effect on a lot of things. As such, it mainly causes waste of natural resources. Additionally, it can further cause hazardous situations such as building structures becoming weak and unstable, accidents caused by corroded parts as well as other unwanted failures, such as cracked pipelines, bridge collapsing, transport vehicle crashes or other catastrophes. It is, therefore, important to check and prevent corrosion at all costs.
Prevention of Corrosion
Preventing corrosion is of utmost importance in order to avoid huge losses. The majority of the structures that we see and use are made out of metals. This includes bridges, automobiles, machinery, household goods like window grills, doors, railway lines, etc. While this is a concerning issue, several treatments are used to slow or prevent corrosion damage to metallic objects. This is especially done to those materials that are frequently exposed to the weather, saltwater, acids, or other hostile environments. Some of the popular methods to prevent corrosion include,
- Electroplating
- Galvanization
- Anodization
- Passivation
- Biofilm Coatings
- Anti-Corrosion Protective Coatings
- Painting and Greasing
- Use of Corrosion Inhibitor or Drying Agents
- Periodic Cleaning of Metal Surface
Read in Detail Here: How Can Corrosion Be Prevented?

Comments