Disproportionation reaction, also called dismutation reaction, is basically a type of redox reaction involving simultaneous reduction and oxidation of atoms of the same element from one oxidation state (OS) to two different oxidation states. Basically, one compound of intermediate oxidation state gets converted to two compounds, one with higher and the other with lower oxidation states. So, a species is simultaneously reduced and oxidised to form two different products.
In general, the term disproportionation reaction can be applied to any desymmetrisation reaction of the following type:
2A → A’ + A”,
This is regardless of whether it is redox or some other type of process.
As for the requirement for a disproportionation reaction to occur, an element undergoing disproportionation must showcase a minimum of 3 different oxidation states. Additionally, the element should be less stable in a particular oxidation state. This allows it to be oxidised as well as reduced to a relatively more stable oxidation state.
The first disproportionation reaction that was studied in detail was,
2 Sn2+ → Sn4+ + Sn
A Finnish chemist named Johan Gadolin examined the reaction using tartrates in the year 1788. It has been recorded in the Swedish version of his paper called ‘Söndring’.
How to Determine if an Element in a Particular Oxidation State Undergoes a Disproportionation Reaction?
If an element’s three oxidation states are written either in increasing or decreasing order, the element in the middle oxidation state will likely be less stable, whereas the higher and lower oxidation states will be relatively more stable. However, the overall process has to be thermodynamically feasible.
More significantly, the Latimer diagrams can be used to predict whether an atom in a particular oxidation state can go through disproportionation or not. If we look at the Latimer diagram, we will find that the unstable species that can undergo disproportionation, which will have a greater positive emf value to its right (for reduction) than the emf to its left (for oxidation).
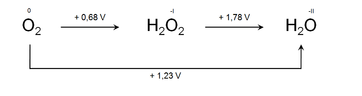
Also Read: Oxidation and Reduction
Disproportionation Reaction Examples
Let us look at a few examples of disproportionation reactions.
Example 1: Decomposition reaction of hydrogen peroxide. Here, oxygen experiences disproportionation.
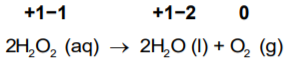
So, the oxygen of peroxide present in the −1 state gets converted to the zero oxidation state in O2 and, at the same time, decreases to the −2 oxidation state in H2O.
Example 2: Upon UV-irradiation, mercury(I) chloride undergoes disproportionation.
Hg2Cl2 → Hg + HgCl2
Example 3: Phosphorous acid, when heated, undergoes disproportionation to produce phosphoric acid and phosphine:
4 H3PO3 → 3 H3PO4 + PH3
Desymmetrisation Reactions
Alternatively, desymmetrisation reactions are also sometimes called disproportionation. This is mainly illustrated by the thermal degradation of bicarbonate:
2HCO-3 → CO2-3 + H2CO3
The oxidation numbers remain constant in this acid-base reaction, and this process is also called autoionisation.
Radical Disproportionation
Also, disproportionation has another variant which is known as radical disproportionation. Here, two radicals tend to form an alkane and alkene.
In other instances, if you look at the Cannizzaro reaction, an aldehyde is converted into alcohol and a carboxylic acid. In the related Tishchenko reaction, the organic redox reaction product is the corresponding ester. In the Kornblum–DeLaMare rearrangement, peroxide is converted to ketone and alcohol.
Other Examples
1. The reaction of chlorine gas with dilute sodium hydroxide results in the formation of sodium chloride, sodium chlorate and water. The ionic equation for this reaction is as follows:
3 Cl2 + 6 OH− → 5 Cl− + ClO3− + 3 H2O
Here, the chlorine reactant is in oxidation state 0, and if we look at the products, the chlorine in the Cl− ion has an oxidation number of −1, where it has undergone reduction. Meanwhile, the oxidation number of the chlorine in the ClO3− ion is +5, which means it has been oxidised.
2. Disproportionation can be seen in the decompositions of numerous interhalogen compounds. For example, bromine fluoride can go through a disproportionation reaction to form bromine trifluoride and bromine.
3BrF → BrF3 + Br2
3. If we study the Boudouard reaction, we can see that carbon monoxide easily disproportionates with carbon and carbon dioxide. While the reaction is an example of the HiPco method that is used for producing carbon nanotubes, high-pressure carbon monoxide undergoes disproportionation when it is catalysed on the surface of an iron particle:
2 CO → C + CO2
4. In nitrogen dioxide, nitrogen has an oxidation state of +4. When this compound is reacted with water, it results in the formation of both nitric acid and nitrous acid, wherein nitrogen has oxidation states +5 and +3, respectively:
2 NO2 + H2O → HNO3 + HNO2
Reverse Reaction
When a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, it is the reverse of disproportionation. This process is known as comproportionation, which is also sometimes referred to as synproportionation.
Disproportionation Reaction Video Lesson
Frequently Asked Questions (FAQs)
What is a disproportionation reaction?
What is the other name for the disproportionation reaction?
Give an example of a disproportionation reaction.
Which reaction is the opposite of the disproportionation reaction?
Example: Ag2+ (aq) + Ag (s) → 2Ag+ (aq)
Comments