National Testing Agency NTA from 2019 onwards, will conduct the JEE (Joint Entrance Examination) all over India. The entrance exam is a platform that provides aspiring candidates admissions into some of the prestigious technical and engineering colleges in the country. Candidates must work tirelessly with intensive hard work and focus on obtaining success in this examination. A few important chapters and some concepts are highly significant as there is a pattern in which these questions repeat every year. Going through these questions will give you a fair idea about your preparation status and help you to utilize the remaining time before the examination.
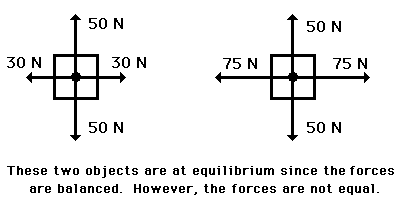
Important Questions For Equilibrium
- What are the important topics in equilibrium?
Ans – Equilibrium does fall under the important topics, which help you in scoring good in JEE. Following are the concepts that should be studied carefully:
- Le Chatelier Principle
- Degree of Dissociation
- Acids and Bases
- Acid-Base Neutralization
- Ionic Product of Water
- Solubility Product
- Salt Hydrolysis
- Buffer Solutions
How to Approach a question in equilibrium?
Q. Calculate the molar solubility of Mg(OH)2 in 1 M NH4Cl solutions.
Books for reference
Refer NCERT textbook for class 11th. This book contains all the details in a very precise manner. A quick view of this can help you to understand the fundamental concepts and their applications. The important points are neatly mentioned in the book making it the most student-friendly book. Some other important reference books include O. P. Tandon and P. Bahadur. For more details download BYJU’S – The Learning App.
Comments