Fructose is a simple ketonic monosaccharide present in many plants, and it is also known as fruit sugar. In plants, it is often bonded to glucose to form the disaccharide sucrose. It is absorbed directly into the bloodstream during digestion.
A French chemist named Augustin-Pierre Dubrunfaut first discovered fructose in the year 1847. Later, in the year 1857, the term was officially coined by an English chemist named William Allen Miller.
| Other Names | Fruit sugar, levulose, d-fructofuranose, d-fructose, d-arabino-hexulose |
| Chemical Formula | C6H12O6 |
| Molar Mass | 180.156 g·mol−1 |
| Density | 1.694 g/cm3 |
| Melting Point | 103 °C (217 °F; 376 K) |
| Water Solubility | ~4000 g/L (25 °C) |
Some key characteristics of fructose: When it is pure and dried, it is white in colour, it is odourless, tastes sweet, and has a crystalline form. It is the most water-soluble among all the different types of sugars. Fructose sugar is usually found naturally in fruits, flowers, trees, honey, berries and rooted vegetables.
As for the chemical properties, fructose is a monosaccharide and also a reducing sugar. The older name of fructose was levulose, as it rotated the plane-polarised light in the left direction. This was the sole reason that it was classified as a laevorotatory compound. On the other hand, glucose rotates the plane-polarised light in the right direction, and is thus categorised as a dextrorotatory compound.
An interesting fact: Bees collect nectar from flowers, which consist of sucrose. Then, they use an enzyme to hydrolyze or break apart the sucrose into its component parts of glucose and fructose.
Also Read: Fructose
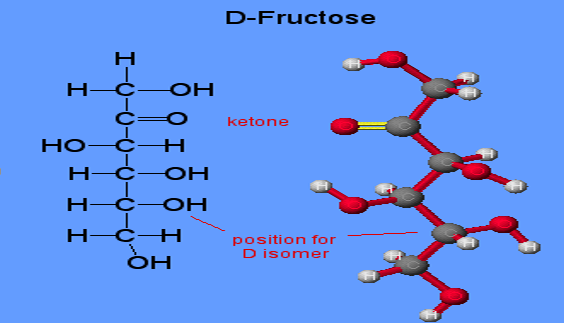
Structure of Fructose
Fructose has a cyclic or chair-like structure. The chair form of fructose is similar to that of glucose, but in the structure of fructose, there are a few exceptions. Fructose has a ketone functional group, and the ring closure occurs from the 2nd carbon position. This results in the rise to a 5-membered ring, or there is a formation of intramolecular hemiacetal in fructose. The OH at the 5th carbon combines with carbon in the 2nd position.
The five-membered ring has four carbons and one oxygen. There is basically a formation of chiral carbon and two arrangements of CH2OH and OH groups. In essence, fructose displays stereoisomerism.
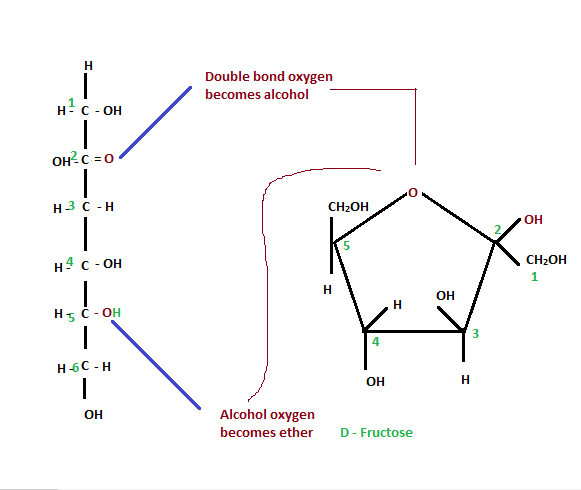
Steps in the Ring Closure (Hemiketal Synthesis)
- The electrons present in the alcohol oxygen are used to bond the carbon at 2nd position to form an ether.
- The hydrogen is transferred to the carbonyl oxygen to form a new OH bond, which further provides a ring to the keto structure of fructose.
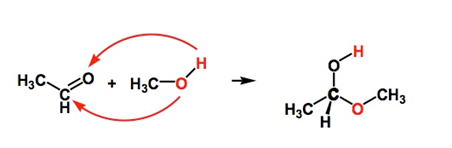
Hemiketal Functional Group
The carbon, which is the centre of the hemiketal functional group, is referred to as anomeric carbon. The hemiketal group has both an ether oxygen and an alcohol group which is attached to two other carbons.
Comparison of Alpha and Beta Structure of Fructose
If the alcoholic group (OH) is present on the same side of the ring as the carbon at 6, then it is a beta structure. The cyclic ring structure leads to an upward projection of the alcoholic (OH) group at carbon 2. If the –OH is present on the opposite side of the ring as the carbon at 6, then the ring structure leads to a downward projection of OH at carbon.
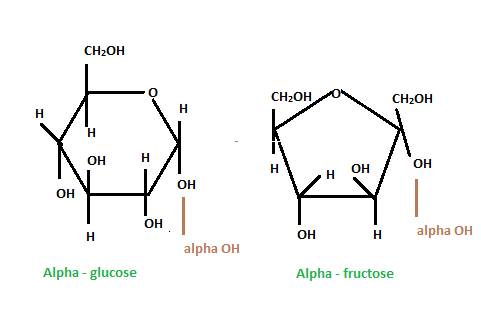
Compare Glucose and Fructose in the Chair Structures
Fructose is identified as a five-membered ring having six-carbon, a hexose, whereas glucose is a six-membered ring with a –OH group on the carbon at the 4th position in a down projection.
Molecular and Structure Formula of Fructose
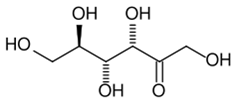
Fructose is a levorotatory monosaccharide, which means it rotates the plane-polarised light in the left direction. The chemical composition of fructose is C6H12O6 but shows different bonding from glucose. Fructose is a hexose, however, it exists as a 5-member hemiketal ring. The hemiketal ring helps with a long metabolic pathway and high reactivity in comparison to glucose.
Isomerism
The configuration of D-fructose is similar in its penultimate carbon as D-glyceraldehyde. Due to the stereometric structure, fructose is much sweeter than glucose.
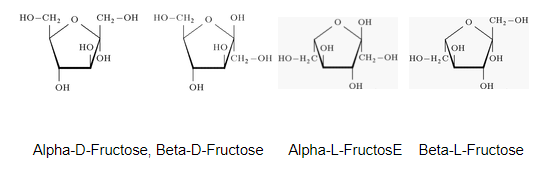
Properties of Fructose
- Fructose is a white crystalline compound.
- It melts at a temperature of 102⁰C.
- Fructose absorbs moisture rapidly and loses it slowly to form hydroxymethylfurfural in the environment in comparison to other sugars.
- It can be fermented anaerobically using yeast or bacteria, by which they are converted into ethanol and carbon dioxide.
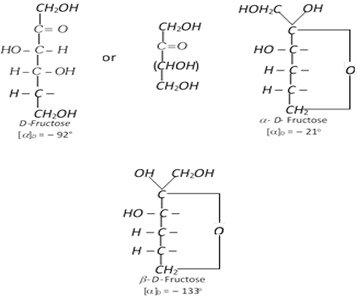
Comparison of Fructose and Glucose in Brief
| PROPERTY | FRUCTOSE | GLUCOSE |
| Molecular formula | C6H12O6 | C6H12O6 |
| Nature | Polyhydroxy ketone | Polyhydroxy aldehyde |
| Melting point | 102⁰C | 146⁰C |
| Optical activity of natural form | Laevorotatory | Dextrorotatory |
| With ethyl alcohol | More soluble | Almost insoluble |
| Reduction | A mixture of sorbitol and mannitol | Sorbitol |
| Molichs reagent | Forms a violet ring | Forms a violet ring |
| Fehlings solution | Gives red precipitate | Gives red precipitate |
| Tollens reagent | Forms silver mirror | Forms silver mirror |
| Phenyl hydrazine | Forms Osazone | Forms osazone |
| Oxidation with bromine water | No reaction | Gluconic acid |
| Oxidation with nitric acid | A mixture of glycollic acid, tartaric acid and tri hydroxyl glutaric acid | Saccharic acid |
| Alcoholic a-naphthol+HCL conc. | Purple colour on boiling | No colouration |
Comparison of Fructose and Sucrose in Brief
| Fructose | Sucrose |
| Monosaccharide | Disaccharide |
| Fructose combine with sucrose to form sucrose | Fructose combine with sucrose to form sucrose |
| Chemical formula – C6H12O6. | Chemical formula – C12H22O11. |
| Molecular weight – 180.16 g/mol | Molecular weight – 342.2965 g/mol |
| Reducing sugar | Non-reducing sugar |
| Melting Point – 03°C (217.40°F) | Melting Point – 186°C (366.80°F) |
| Boiling point – 440°C (824°F) | Boiling point – 669.431 °C |
| Density: 1.69 g/cm³ | Density: 1.59 g/cm³ |
Uses of Fructose
Some common uses of fructose are listed below:
- It helps in enhancing the taste of food and beverages.
- Crystalline fructose (natural sweetener) is used in energy drinks, chocolate milk, cereals, yoghurts and many other beverages and low-calorie food.
- HFCS (High Fructose Corn Syrup) products are a combination of glucose and fructose.
- High fructose corn syrup is also used as a sweetener in many pediatric and adult medicines, such as cough suppressants and decongestant liquids.

Comments