Nuclear fission is the process of splitting a heavy nucleus, such as uranium or plutonium, in two smaller nuclei of nearly the same mass. During this process, the unstable radioactive nucleus is split into two smaller nuclei. Nuclear fission can occur spontaneously in some cases or can be induced by the bombardment on the nucleus with a variety of particles (e.g., protons, or neutrons or alpha particles) or by gamma rays radiation.
- During this fission process, a huge amount of energy is produced further giving rise to radioactive elements as well as the release of many neutrons.
- These neutrons can further induce chain fission reaction in the nucleus of the uranium or plutonium and release more neutrons.
- This can result in an uncontrolled chain reaction till all the starting material is exhausted where a large amount of energy is also produced.
If such reactions can be controlled in a nuclear reactor, these chain reactions can be harnessed to meet the electricity needs of society. On the other hand, such uncontrolled reactions can lead to the formation of atom bombs, which can be very devastating.
The modern “Atomic Age” is attributed to the discovery and developments in the field of nuclear fission. This has its own boon and bane. Its judicious usage and development can help us to develop at a great speed and in a sustainable manner, but if it falls in the wrong hands it can cause a great threat to humanity. However, there is still a great scope of development and research in this field, and many more questions are yet to be answered.
Nuclear Fission Energy
As mentioned above, the splitting of neutrons results in the release of a large amount of energy. During this process, there is a strong repulsion force between the protons. But, they are also bound together by the strong nuclear force. Typically, each proton applies a repulsion force of 20N on every other proton, and that is equal to the force of a hand resting on a person’s lap. This is really a very large force for these atomic particles.
- Due to such large force inside the small nucleus, it leads to the production of a large amount of energy and is enough to cause a considerable reduction in mass. This implies that the total mass of each of the fission fragments is less than the mass of the starting nucleus. Here the missing mass is called the mass defect.
- It is easy to understand the amount of energy that binds all the nuclei together. Every nucleus has this binding energy except hydrogen. Therefore, the binding energy available to each nucleon is simply called the binding energy per nucleon. The same amount of energy is actually required per nucleon to split a nucleus.
The products formed after fission are more stable means further splitting is very hard. As this binding energy for fission products is very high, the nucleonic mass becomes lower. The result of this large binding energy and lower mass results in the release of energy. Nuclear binding energy and mass defects are also used interchangeably.
Basics of Nuclear Fission
When a nucleus fissions reaction takes place, the neutron breaks the target nucleus into further smaller products. These fission products are nearly equal to half the original mass. Two or three neutrons are also emitted.
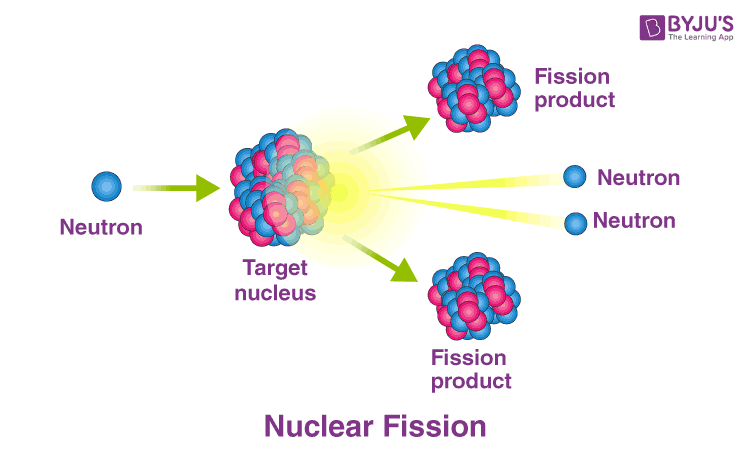
Interestingly, the total sum of the masses of products is less than the initial nuclei mass. This ‘missing’ mass (which is about 0.1 % of the initial mass) is converted into energy according to Einstein’s equation: E = mc2. Fission always occurs when neurons are bombarded on a nucleus of a heavy atom, or it can happen spontaneously.
Nuclear Fission Reaction Mechanism
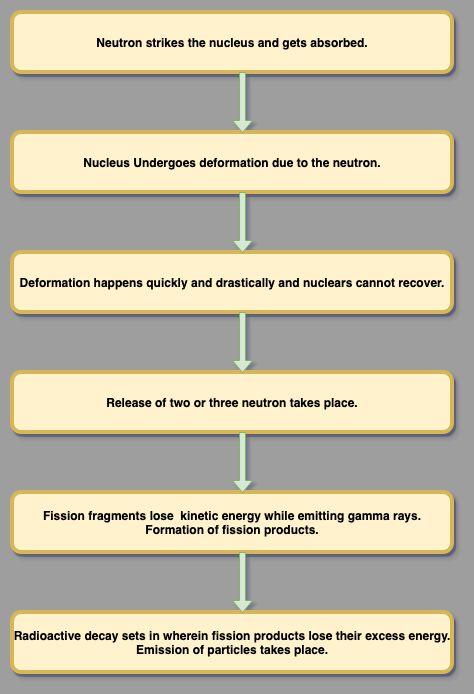
Here is a quick step-by-step process of the reaction mechanism in nuclear fission.
Nuclear Fission Reactor
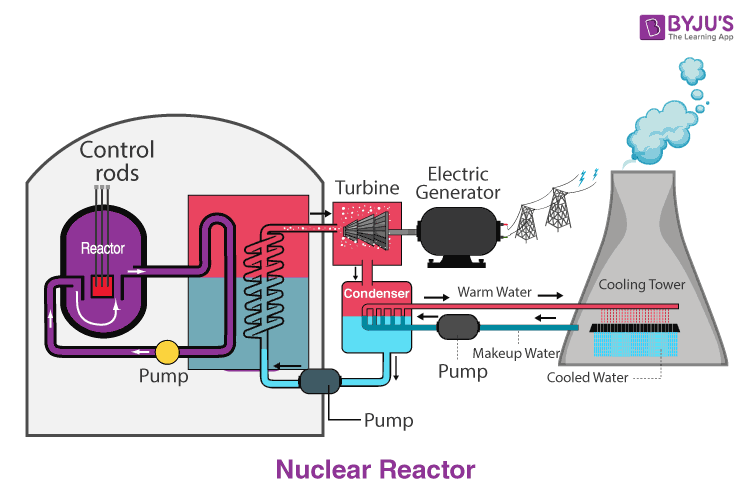
A nuclear reactor is the most important part of a nuclear power plant. This is the place where nuclear chain reactions occur that produce energy by fission. The heat thus produced can be used to produce electricity.
The main purpose of a reactor is to contain and control energy released. Uranium is used as the nuclear fuel in the reactors. The uranium is treated with ceramic pellets and they are sealed in the form of metal tubes called fuel rods. Generally, about 200 such rods are assembled together to form a fuel assembly. When a hundred of such assemblies are assembled together, it is called the core.
The fuel rods are dipped in water in the reactor, which functions as both a coolant and moderator. The job of the moderator is to slow down the neutrons produced by fission to control the chain reaction. Control rods may be immersed in the reactor core to reduce the reaction rate or pulled out to increase the same. The heat produced by such reactions converts the water into steam, which is further converted into carbon-free electricity by the help of turbines. Nuclear Fission Reactor is as below:
Advantages and Disadvantages of Nuclear Fission
Advantages
Clean Alternative Source of Energy
Nuclear fission is one of the most researched and well-known topics of nuclear technology. This has been utilised as a very clean alternative source of energy. It will not be a hyperbole if we tell it as “the energy source of the future”. This technology is now achieving perfection and excellence and emerging at a very fast pace compared to other alternative sources of energy.
Fulfil the Needs of the Present and Future Generations
An enormous amount of energy is produced by the process of nuclear fission, which can be utilised to fulfil the needs of next-generation high tech cities and industries. As this is a very rapid reaction, energy can be produced depending on the requirement, with greater reliability.
Reduced Threat from Greenhouse Gases Production and Global Warming
In contrast to fossil fuels, which cause serious damage to the environment, nuclear fission produces energy without releasing greenhouse gases into the atmosphere, which reduces the effects of global warming and even helps fight pollution.
Extremely Low Operation Cost
The cost of operation is very low once the nuclear power plant is commissioned. The costs in operation are only the payment of workers to run the plant and the cost of the raw materials required.
Disadvantages
Higher Risk of Radiation Exposure
The radiation emitted during the fission reaction is extremely harmful to humans and animals. The workers who are exposed while working at nuclear power plants are at great risk of radiation poisoning, cancer and other diseases associated with radiation.
Highly Vulnerability
High vulnerability is a potential risk that is involved in nuclear power plants. The enormous energy produced in a fission reaction can be utilised to create nuclear weapons. Any small accident at a nuclear plant can cause huge damage, adversely affecting the lives of millions.
Radioactive Contamination Risk
The waste that is released from the nuclear power plant is highly radioactive and harmful for all living beings. There are high chances of water contamination from these plants, which can cause serious diseases and death in living beings.
High Cost of Plant Commissioning
To build a nuclear power plant requires a huge investment, and this is due to the latest technologies and safety measures that are required to run it properly.
Important Questions
Question.1: Neutrons can be emitted by the process of?
a) Nuclear fission
b) Inverse beta Decay
c) Nuclear fusion
d) Spontaneous Fission
Answer: a)
In nuclear fission, a heavy nucleus is divided into two or more smaller nuclei. This results in mass decrease and large energy release and neutrons are also emitted in the process. Two or three neutrons are ejected per nuclei, which are called fission elements.
Question.2: Neutrons with lower energy are used for nuclear fission. This is done
a) to increase the rate of reaction
b) to maintain safety standards
c) for a sustained reaction process
d) for prevention of wastage of nuclear fuel
Answer: c)
The kinetic energy of neutrons decreases due to collisions among themselves. Thus, for a sustained reaction, these neutrons with lower energy should be capable of causing fission. Only neutrons can result in a sustained reaction because fission further produces neutrons only.
Question.3: What would happen when neutrons are on a nucleus of an atom of U235?
a) One electron is let out
b) The mass number of the atom increases
c) Nucleus becomes unstable
d) U236 isotope is formed
Answer: d)
When neutrons are bombarded, one neutron is absorbed by the nucleus of U235 atom, forming a U236 isotope. This isotope is highly unstable which decays within one-millionth of a second and divides into two equal parts producing 200MeV of energy.
Question.4: The energy released in fission is a form of __________
a) Thermal Energy
b) Kinetic Energy
c) Heat Energy
d) Light Energy
Answer: b)
The energy released in fission is mostly in the form of kinetic energy and is absorbed by fission products. The fission products are neutrons and gamma radiation. As the neutrons collide, the kinetic energy is transformed into heat energy.
Watch the below Video for More Modern Physics Important JEE Main Questions

Frequently Asked Questions on Nuclear Fission
What is nuclear fission?
The process in which a heavy nucleus bombarded by a neutron splits into lighter nuclei of comparable masses, releasing two or three neutrons and energy, is called nuclear fission.
In what form is the energy released in nuclear fission reactions?
The energy released in nuclear fission appears as kinetic energy of fission fragments, neutrons in the form of gamma rays, heat energy and light energy.
How is nuclear fission initiated in a nuclear reactor?
Stray neutrons usually initiate nuclear fission.
What is a nuclear reactor?
A nuclear reactor is a device in which a self-sustained yet controlled nuclear chain reaction takes place.

Comments