Potassium permanganate, which is also commonly referred to as KMnO4, is a chemical compound that is used in many industries for oxidising functionality. This compound is also sold commercially, mostly for medicinal purposes. It is available in the form of crystals, powder and tablets. While this is one of the most discussed topics in chemistry, we will understand everything about the compound in detail below.
The potassium permanganate formula is written as KMnO4. This inorganic compound belongs to the category of salt and consists of two ions, namely K+ and MnO-4 ions. Some of its key properties include its oxidising and solubility properties. KMnO4 is a strong oxidising agent, and most of its uses are based on this property. It is also easily soluble in water and usually forms a pinkish or purple solution when added to water. Below, we will talk more comprehensively about this chemical compound.
| IUPAC Name | Potassium permanganate |
| Chemical Formula | KMnO4 |
| Molar Mass | 158.034 g/mol |
| Density | 2.7 g/cm3 |
| Melting Point | 240 °C (464 °F; 513 K) |
Discovery of KMNO4
The traces of KMnO4 was first found when a German-Dutch chemist named Johann Rudolf Glauber started his experiments by mixing two compounds, namely pyrolusite, which is a mineral consisting of manganese dioxide and potassium carbonate. He dissolved the mixture in water, which led to the formation of a green solution. This was potassium manganate. Interestingly, the colour of the solution slowly changed from blue to violet and red. This experiment represented the first production of KMnO4.
A more prominent discovery was made by a chemist from London, Henry Bollmann Condy. In his case, he fused pyrolusite with sodium hydroxide (NaOH) and dissolved it in water. This led to the production of a solution with several characteristics, and the primary one was the disinfectant property. The solution was patented and used commercially. It was known as ‘Condy’s fluid’. However, there was one drawback as the solution was found to be unstable. This defect was corrected with the use of potassium hydroxide (KOH) instead of NaOH. Moreover, potassium permanganate crystals could now be obtained from the solution. Later, these crystals were known as ‘Condy’s powder’ or ‘Condy’s crystals’.
Preparation of KMnO4
Industrially, KMnO4 is prepared using manganese dioxide. As stated above, this chemical compound is reacted with alkali metal hydroxide, which is potassium hydroxide. The mixture is then heated in the air at high temperatures. When the reaction is completed, potassium manganate is formed.
2 MnO2 + 4 KOH + O2 → 2 K2MnO4 + 2 H2O
After the formation of potassium manganate, it goes under electrolytic oxidation in alkaline media to form permanganate.
2 K2MnO4 + 2 H2O → 2 KMnO4 + 2 KOH + H2
Alternatively, potassium permanganate can also be formed using a chemical oxidation process. It involves treating potassium manganate either with ozone or chlorine. K2MnO4 is oxidised to KMnO4 by bubbling CO2 or Cl2 or ozone.
3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3
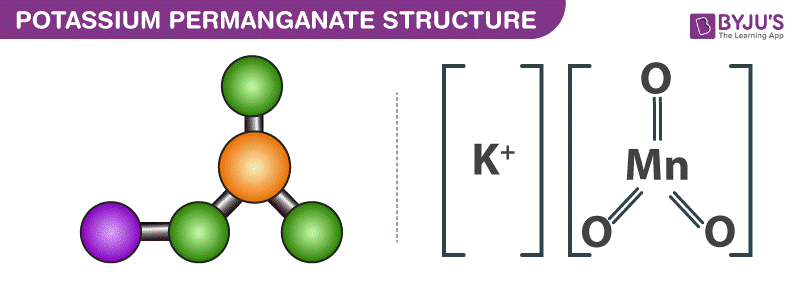
KMnO4 Structure
When KMnO4 is in the solid state, it has an orthorhombic crystal structure, where each MnO4− centre is arranged in a tetrahedral geometry.
Potassium Permanganate Properties
We will look at the physical and chemical properties of KMnO4 below.
Physical Properties
- KMnO4 is usually in the form of a crystalline solid.
- It is odourless.
- KMnO4 colour is described to be purple or magenta.
- KMnO4 molar mass is 158.034 g/mol.
- It has a density of 2.7 g/cm3.
- Its melting point is 240 °C (464 °F; 513 K).
Chemical Properties
- KMnO4 has high solubility and easily dissolves in water.
- It acts as a strong oxidising agent.
- It can react violently with concentrated acids, such as sulphuric acid and various alcohols. It can even produce fire when reacted with substances like glycerin.
KMnO4 Reactions
KMnO4 can be used to oxidise a lot of organic compounds. It can also be used to oxidise carbon atoms, mostly with weak bonds. It can react with alcohols, alkenes, aldehydes, alkynes and aromatic side chains.
- Aldehydes are generally oxidised to carboxylic acids.
- Alcohols are oxidised to carbonyls.
- Potassium permanganate can be used to oxidise alkenes to glycols.
- In alkynes, there is a formation of diones.
KMnO4 Uses
Potassium permanganate uses are mostly centred around its oxidising property. It is also used widely because there is no generation of toxic byproducts.
- KMnO4 is used extensively as an antiseptic to treat different kinds of fungal infections, ulcers, wounds and other skin conditions.
- It is used in water treatment mainly to remove minerals, such as iron and hydrogen sulfide, and control organisms, such as zebra mussels.
- This chemical compound is also used to remove rust that may be found in water pipes and equipment.
- Another major use of potassium permanganate is in the synthesis of organic compounds.
- It is used in the preservation of fruits.
- It is found in survival kits. It can be used to start a fire easily.
- It is also used in rocket propellant.
- KMnO4 is used in treating fish diseases and parasites.
Adverse Effects of KMnO4
We will understand some adverse effects of KMnO4. Normally, the concentrated solution of potassium permanganate is usually corrosive in nature. It can cause irritation and burns on the skin as well as damage to the membranes in the eyes, throat, nose, etc. It also leaves a coloured stain on the skin and nails. Ingestion of potassium permanganate can lead to abdominal pain, vomiting, burns in the throat, shortness of breath, cardiovascular collapse, kidney damage and sometimes, fatality.
Frequently Asked Questions – FAQs
What are the uses of potassium permanganate?
How is KMnO4 prepared?
2K2MnO4 + 4HCl → 2KMnO4 + MnO2 + H2O + 4KCl

Comments