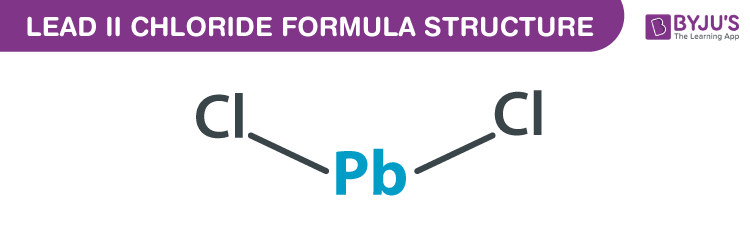
Lead (II) chloride formula, also named as Lead dichloride formula or Plumbous chloride formula is discussed in this article. It is an inorganic chloride which consists of two chlorine atoms and one lead atom. The chlorine atoms bind to the central lead atom covalently. The molecular or chemical formula of Lead (II) chloride is PbCl2.
Lead dichloride is a crystalline solid colourless and odourless. It is insoluble in water but dissolves in solutions which contain chloride ions. When it reacts with molten sodium nitrite it generates lead(II) oxide.
Lead (II) chloride Formula Structure
Properties Of Lead (II) chloride Formula
| Chemical formula | PbCl2 |
| Molecular weight | 278.10 g/mol |
| Density | 5.85 g/cm3 |
| Boiling point | 950 °C |
| Melting point | 501 °C |
Use Of Lead (II) Chloride
- It is widely used in making ceramics, infrared transmitting glass.
- In HCl service, to produce ornamental glass, used in white paint, etc.
To learn more about Lead (II) chloride formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Plumbous chloride for free.
Comments