Lithium Chloride formula, also known as Chlorolithium formula or Lithiumchlorid formula is explained in this article. Lithium Chloride is formed by one lithium atom and one chlorine atom. In the year 1940, for a short period of time, this compound was produced as a salt substitute. Due to its toxic effects, it was prohibited immediately. The molecular or chemical formula of Lithium Chloride is LiCl.
Lithium Chloride occurs as a colourless to white hygroscopic and deliquescent powder or crystals. It has sharp saline like a taste. Chlorolithium can be produced by treating lithium carbonate (Li2CO3) with hydrochloric acid (HCl). It can also be synthesized by high exothermic reaction of lithium metal with anhydrous hydrogen chloride gas or chlorine. Anhydrous LiCl is obtained from the hydrate by heating it with a stream of hydrogen chloride (HCl).
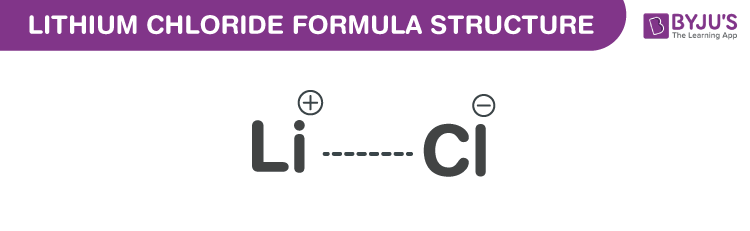
Lithium Chloride Formula Structure
Properties Of Lithium Chloride
| Chemical formula | LiCl |
| Molecular weight | 42.39 g/mol |
| Density | 2.068 g/cm3 |
| Boiling point | 1,382 °C |
| Melting point | 605 – 614 °C |
Chlorolithium is mainly used in the manufacturing of lithium metals by the method of electrolysis. In this method, Lithium Chloride or potassium chloride is melted at 450 °C. LiCl is also had wide application as a brazing flux for aluminium used in automobile parts.
To learn more about Lithium Chloride formula from the expert faculties at BYJU’S, register now!
Comments