Magnesium bromide formula is discussed in this article. It is a chemical compound made of bromine and magnesium. The molecular or chemical formula of Magnesium bromide is MgBr2.
What Is The Formula For Magnesium Bromide?
In its anhydrous form, it is hygroscopic hexagonal crystal white in colour. In its hexahydrate for, it is colourless monoclinic crystal. It can be prepared by the following method
- Treating hydrobromic acid (HBr) with magnesium oxide (MgO) and crystallizing the product.
- It can also be synthesized by reacting magnesium carbonate (MgCO3) and hydrobromic acids (HBr).
On completing this it is evaporated and the solid leftover is collected. It is soluble in water as well as alcohol. It is naturally found in some minerals such as carnallite and bischofite.
Magnesium bromide Formula Structure
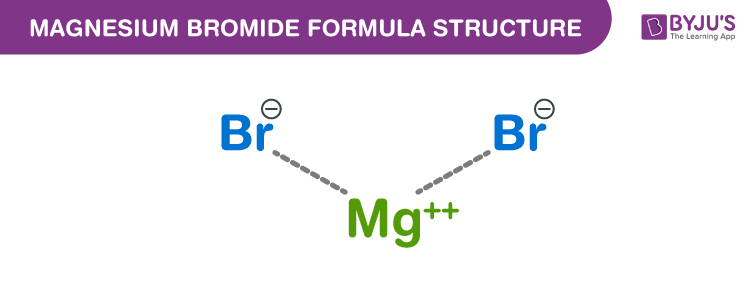
Properties Of Magnesium bromide Formula
| Chemical formula | MgBr2 (anhydrous)
MgBr2·6H2O (hexahydrate) |
| Molecular weight | 184.113 g/mol (anhydrous)
292.204 g/mol (hexahydrate) |
| Density | 3.72 g/cm3 (anhydrous)
2.07 g/cm3 (hexahydrate) |
| Melting point | 711 °C
172.4 °C (hexahydrate) |
| Boiling point | 1,250 °C |
Use Of Magnesium Bromide
- It is used as a tranquillizer
- It is used as a catalyst
- It is used to analyse regiospecific of triglycerols.
- It is widely used as an anticonvulsant for the treatment of nervous disorders.
- It is used as a mild sedative.
To learn more about Magnesium bromide formula from the expert faculties at BYJU’S, register now! Also, you can download notes for free.
Comments