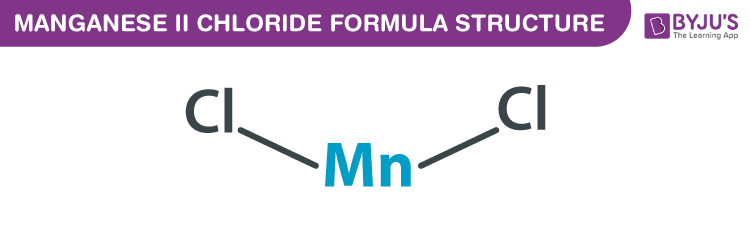
Manganese (II) chloride formula, also named as Potassium biphosphate formula or Monopotassium Phosphate formula is discussed in this article. The molecular or chemical formula of Manganese (II) chloride is MnCl2.
Manganese chloride is synthesized by treating manganese(IV) oxide with concentrated hydrochloric acid (HCl). It is widely used in the manufacturing of dry cell batteries. Also, it acts as a precursor to the antiknock compound MMT (Methylcyclopentadienyl manganese tricarbonyl)
It appears as a pink solid in its tetrahydrate form. It is soluble in water, ethanol but insoluble in ether. It is slightly soluble in ethanol.
Manganese (II) chloride Formula Structure
Properties Of Manganese (II) chloride Formula
| Chemical formula | MnCl2 |
| Molecular weight | 125.844 g/mol (anhydrous)
161.874 g/mol (dihydrate) 197.91 g/mol (tetrahydrate) |
| Density | 2.977 g/cm3 (anhydrous)
2.27 g/cm3 (dihydrate) 2.01 g/cm3 (tetrahydrate) |
| Melting point | 654 °C (anhydrous)
135 °C (dihydrate) 58 °C (tetrahydrate) |
| Boiling point | 1,225 °C (anhydrous) |
Safety Measures
- When exposed to manganese fumes or dust for a long duration, it causes manganese poisoning or magnesium poising.
To learn more about Manganese (II) chloride formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Manganous chloride for free.
Comments