Manganese(II) sulfate formula, also named as Manganese sulphate formula or Manganous sulfate formula is discussed in this article. It is a metal sulfate where the oxidation state of is manganese is +2. The molecular or chemical formula of Manganese(II) sulfate is MnSO4.
In its anhydrous form, it appears as a white crystal. As a hydrate, it appears pale pink solid. It functions as a nutraceutical. Minerals of manganous sulfate are very rare in nature. They always are available as hydrates.
- Monohydrate is called Szmikite
- Tetrahydrate is called Ilesite
- Hexahydrate is called Chvaleticeite
- Pentahydrate is called Jokokuite
- Heptahydrate is called Mallardite
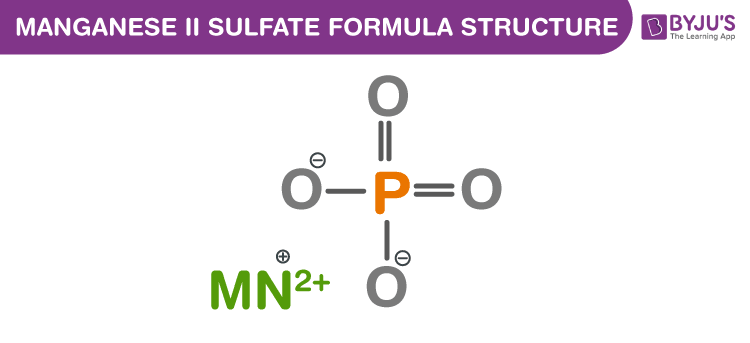
Manganese (II) sulfate Formula Structure
Properties Of Manganese (II) sulfate Formula
| Chemical formula | MnSO4 |
| Molecular weight | 151.001 g/mol (anhydrous)
169.02 g/mol (monohydrate) 223.07 g/mol (tetrahydrate) 277.11 g/mol (heptahydrate) |
| Density | 3.25 g/cm3 (anhydrous)
2.95 g/cm3 (monohydrate) 2.107 g/cm3 (tetrahydrate) |
| Melting point | 710 °C (anhydrous)
27 °C (tetrahydrate) |
| Boiling point | 850 °C (anhydrous) |
Use Of Manganese (II) sulfate
- Manganese (II) sulfate undergo electrolysis process to produce manganese dioxide (electrolytic manganese dioxide) which are used in dry-cell batteries.
To learn more about Manganese (II) sulfate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Manganous sulfate for free.
Comments