Mercury II Nitrate is a white crystalline solid that is toxic and soluble in water. Reacting hot concentrated nitric acid with mercury metal, Mercury II Nitrate is obtained. When heated, it decomposes into nitrogen and oxygen. Prolonged exposure to heat or fire may lead to an explosion. Due to its toxicity, it is difficult to get hold of it in the market. In this short piece of article, learn the mercury II Nitrate formula, its chemical structure, its properties along with its uses.
Mercury II Nitrate Properties
| Properties of Mercury II Nitrate Properties | |
| Name | Mercury II Nitrate |
| Also Known as | Mercuric Nitrate |
| Appearance | Colourless crystals or white powder |
| Chemical Formula | Hg(NO3)2 |
| Melting Point | 79 °C |
| Density | 4.3 g/cm³ (monohydrate) |
| Molar Mass | 324.7 g/mol (anhydrous) |
| Solubility in Water | Soluble |
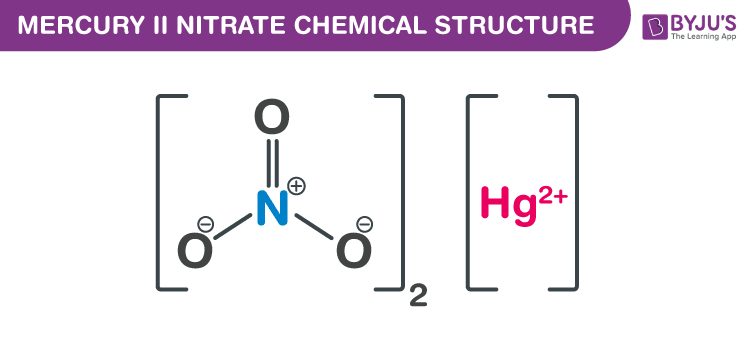
Mercury II Nitrate Chemical Structure
Mercury II Nitrate Uses
- Used in mercuration reactions
- It serves as nitrification and analytical reagents used in laboratories
- Used in the preparation of mercury fulminate
To learn more about such chemistry topics register to BYJU’S now!
Comments