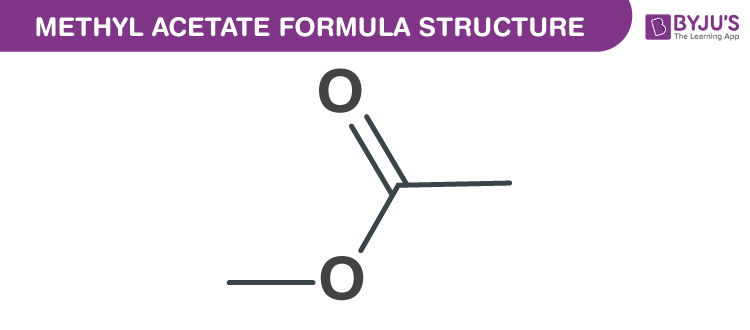
Methyl acetate formula, also named as Methyl acetic ester formula or Methyl ethanoate formula is discussed in this article. It is a carboxylate ester as well as a flammable liquid. The molecular or chemical formula of Methyl acetate is C3H6O2.
It is a colourless volatile liquid with a pleasant odour and fleeting fruity like the taste. It is formed from the condensation of acetic acid and methanol. At 57 °C it is a colourless, and flammable liquid which is used as a solvent for oils and many resins.
It naturally occurs in apple. And can be used as a flavouring ingredient. It is also found in banana, grape, etc. This ester compound can be synthesized from acetic acid and methanol in the presence of strong H2SO4 in an esterification reaction.
Methyl acetate Formula Structure
Properties Of Methyl Acetate
| Chemical formula | C3H6O2 |
| Molecular weight | 74.079 g/mol |
| Density | 0.932 g/cm3 |
| Boiling point | 56.9 °C |
| Melting point | −98 °C |
Use Of Methyl Acetate
- It is widely used as a volatile low toxic solvent in paints, nail paint removers, and glues.
To learn more about Methyl acetate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Methyl ethanoate for free.
Comments