One of the best ways to understand the topics covered under Chemistry is to refer to the MP Board Class 11 Chemistry Syllabus. The Chemistry for Class 11 MP Board covers a wide range of interesting topics like the structure of atoms, classification of elements, molecular structure, states of matter, redox reactions, organic chemistry and more. Hence, the Madhya Pradesh Board Class 11 Chemistry Syllabus is indeed very helpful for students who wish to get details of the course, like the time allotted to take per unit or subtopics under it and so.
The Class 11 Chemistry is the foundation for students who will further continue their higher studies or career in subjects where Chemistry is required. The MP Board Class 11th Chemistry Syllabus will start with an introduction to the basic concepts of Chemistry, the importance of classifying elements, various concepts of the system and so on, finally conclude with teaching about environmental pollution.
Learn the Class 11 Chemistry thoroughly, and students will have a strong foundation for their future studies and career ahead. Class 11 Chemistry is also the basis for many of the competitive exams. Class 12 students who have done well in their Class 11 and know the MP Board Class 11 Chemistry Syllabus 2021-2022 and the subject will find it relatively easy to do their exams.
MP Board Class 11 Chemistry Syllabus 2021-2022 PDF
Find screenshot of the chapter details in Hindi from the current academic year:
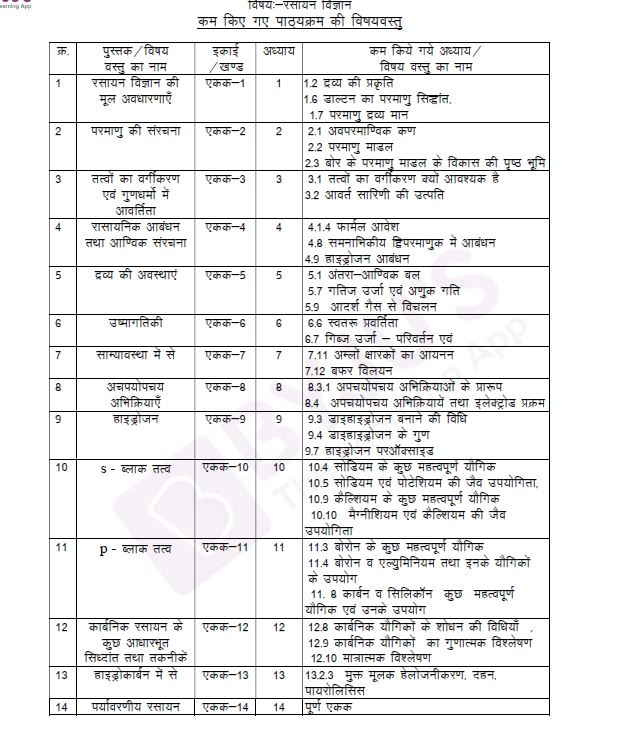
Details of the Chapters with deleted portions for the last academic year 2020-21 are also given below for reference.
Unit I Some Basic Concepts of Chemistry
Unit II Structure of Atom
Unit III Classification of Elements and Periodicity in Properties
Unit IV Chemical Bonding and Molecular Structure
Unit V States of Matter: Gases and Liquids
Unit VI Chemical Thermodynamics
Unit VII Equilibrium
Unit VIII Redox Reactions
Unit IX Hydrogen
Unit X s -Block Elements
Unit XI p -Block Elements
Unit XII Organic Chemistry: Some Basic Principles and Techniques
Unit XIII Hydrocarbons
Unit XIV Environmental Chemistry
Students can also download other study material and resources for MP Board from BYJU’S.

Comments