MSBSHSE Solutions For SSC (Class 10) Science Part 1 Chapter 9 Carbon Compounds are provided here for the students to practice. Students can use these solutions to prepare most efficiently for their exam. Maharashtra Board Solutions for Class 10, designed by our subject experts are available for students. These solutions also facilitate precise and clear understanding of all the key concepts and topics that were covered in the chapter.
This chapter mainly focuses on the concept of Carbon Compounds and has detailed step-by-step solutions for the questions covering the chapter. The chapter deals with Bonds in carbon compounds, Hydrocarbons, Functional Groups and homologous series, Chemical Properties of Carbon Compounds and related topics. Carbon: A Versatile Element, Nomenclature of Carbon compounds as well as Macromolecules and Polymers are other topics discussed in this chapter.
These solutions of MSBSHSE for Class 10 (SSC) come with detailed explanations of the exercises to be found in the Maharashtra Board Science Textbooks for SSC Part 1. The Maharashtra State Board Solutions for Chapter 9 Science Part 1 are easily accessible here. Students can easily use it as a reference tool to quickly revise all the topics for the exam.
Maharashtra Board SSC (Class 10) Science Part 1 Chapter 9- BYJU’S Important Questions & Answers
1. Explain the term Structural isomerism with examples.
Answer: Structural isomerism is the phenomenon where compounds have different structural formulae even if they have the same molecular formula. Learn more about structural isomerism from here.
2. What is the IUPAC name for the structural formula CH3-CH2-CH2-CH3?
Answer: The IUPAC name for the structural formula C4H10 is given here. Two isomeric compounds with a straight chain or branched chain in their structural formulae are possible for the molecular formula C4H10. The difference and interrelationship in them were indicated by naming them as n-butane (normal-butane) and i- butane (iso-butane).
3. Identify the type of the following reactions of carbon compounds.
a. CH3-CH2-CH2-OH→CH3-CH2-COOH
b. CH3-CH2-CH3→ 3CO2 + 4H2O
Answer: a. Oxidation Reaction (acidic KMnO4)
b. Combustion Reaction
4. What causes the existence of a very large number of carbon compounds?
Answer: The molecular masses of carbon compounds range up to 1012, allowing for carbon atoms to come together in a large number and form huge molecules. This unique property of carbon is due to the peculiar nature of the covalent bonds formed by carbon, creating many compounds. Carbon has a unique ability of forming strong covalent bonds with other carbon atoms, resulting in formation of big molecules. This property of carbon is called catenation power.
5. What is meant by vinegar and gasohol? What are their uses?
Answer: Vinegar is a kind of liquid that contains ethanoic acid, the acetic acid. Vinegar is produced by the fermenting ethanol via ethanoic acid in the presence of bacteria. Vinegar is used as a salad dressing, as a household cleaning agent, used to make pickles and so on.
Meanwhile, Gasohol is a combination of 90% alcohol and 10% Ethyl alcohol, also known as an alternative fuel or motor fuel. Gasohol is used in the automobile industry, used as common gasoline, also as a flexible fuel vehicle and more.
6. As the valence shell of carbon contains 4 electrons, what are the many alternative routes to attain a noble gas configuration? Which route do carbon atoms take and why?
Answer: There are many routes to attain a noble gas configuration as given below:
1. Attain configuration of noble gas helium (He) by losing all the four valence electrons one after the other. In this process, the net positive charge on the carbon atom increases during the loss of every electron. In such cases more energy is required to lose the next electron. This makes the task more difficult. Moreover, the C4+ cation ultimately formed in this process becomes unstable despite its noble gas configuration, because it has a small size with high net charge. Therefore, carbon atoms do not adapt this method to attain a noble gas configuration.
2. Now gas neon (Ne) in order to attain the stable configuration accept one by one the four electrons in the valence shell. In this method the net negative charge on the carbon atom increases while accepting every new electron. Hence, more energy is required for accepting the next electron by overcoming the increasing repulsive force making the task more and more difficult. Moreover, the C4- anion ultimately formed would be unstable in spite of its noble gas configuration, as it would have a small size with high net charge, making it difficult for the nuclear charge +6 to hold 10 electrons around it. For this reason, carbon atoms do not take this route to attain a noble gas configuration.
3. Now, take the case of Neon, where it shares four electrons of valence shell with four valence electrons of other atoms. This method enables two atoms to share valence electrons with each other. Valence shells of both the atoms overlap to accommodate the shared electrons, As a result, both the atoms attain a noble gas configuration without generating any net charge on them, essentially making the atoms electrically neutral. Because of these factors atoms attain stability. Therefore, carbon atoms adopt this route to attain a noble gas configuration.
7. Draw the electron-dot structure and line structure of methane molecules.
Answer:
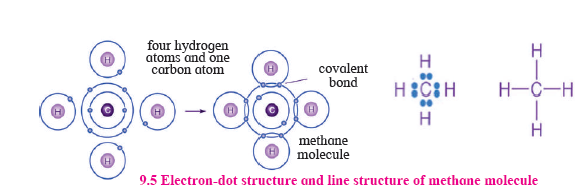
8. What is a homologous series?
Answer: Homologous series are the series of compounds that are formed by joining the same functional group in the place of a particular hydrogen atom on the chains having sequentially increasing length. There are various homologous series based on the functional group. Examples are homologous series of alcohols, homologous series of carboxylic acids, homologous series of aldehydes and so on.
9. Take 2-3 ml ethanol in a test tube, add 5 ml sodium carbonate solution to it and warm the mixture by holding the test tube on the burner for a while. Do drop rise addition of a dilute solution of potassium permanganate to this warm mixture with stirring. Does the typical pink colour of potassium permanganate stay as it is in addition? Does the pink colour stop vanishing and stays on after some time of the addition process?
Answer: During this activity, ethanol gets oxidised by alkaline potassium permanganate to form ethanoic acid. Only certain bonds in the vicinity of the functional group take part in this reaction. When you add the pink coloured solution of potassium permanganate to ethanol, the pink colour disappears in the beginning, because potassium permanganate is used up in the oxidation reaction. At a certain point of the addition, oxidation of all the quantities of ethanol in the test tube is complete. If the addition of potassium permanganate continues beyond this point, it is not used up and becomes excess. The pink colour of this excess potassium permanganate does not vanish, but stays as it is.
Students are recommended to go through all the topics thoroughly and prepare more effectively for the Maharashtra State Board exam. The resources we offer include textbooks, question papers and syllabus.
Frequently Asked Questions on Maharashtra State Board Solutions for Class 10 Science Part 1 Chapter 9 Carbon Compounds
How do I access Maharashtra State Board Class 10 Science Part 1 Solutions Chapter 9?
We have provided the solutions as a scrollable PDF on the webpage, and we have also mentioned the clickable link for the students to download the PDF versions for future reference. Meanwhile, we also make available the questions and the solutions online on our webpage.

Comments