Flashcards for NEET Chemistry are designed to boost your NEET preparation. Find below flashcards for the chapter “Chemical Kinetics”. These flashcards are prepared as per the NEET syllabus. These are helpful for aspirants of NEET and other exams, during last-minute revision. It covers all the important points that are frequently asked in the exam. Check BYJU’S, for the full set of Flashcards and Study material for NEET Chemistry.
Download PDF of NEET Chemistry Flashcards for Chemical Kinetics
|
Name of the NEET Sub-section |
Topic |
Flashcards Helpful for |
|
Chemistry |
Chemical Kinetics |
NEET Exams |
|
Chemical Kinetics |
|
|
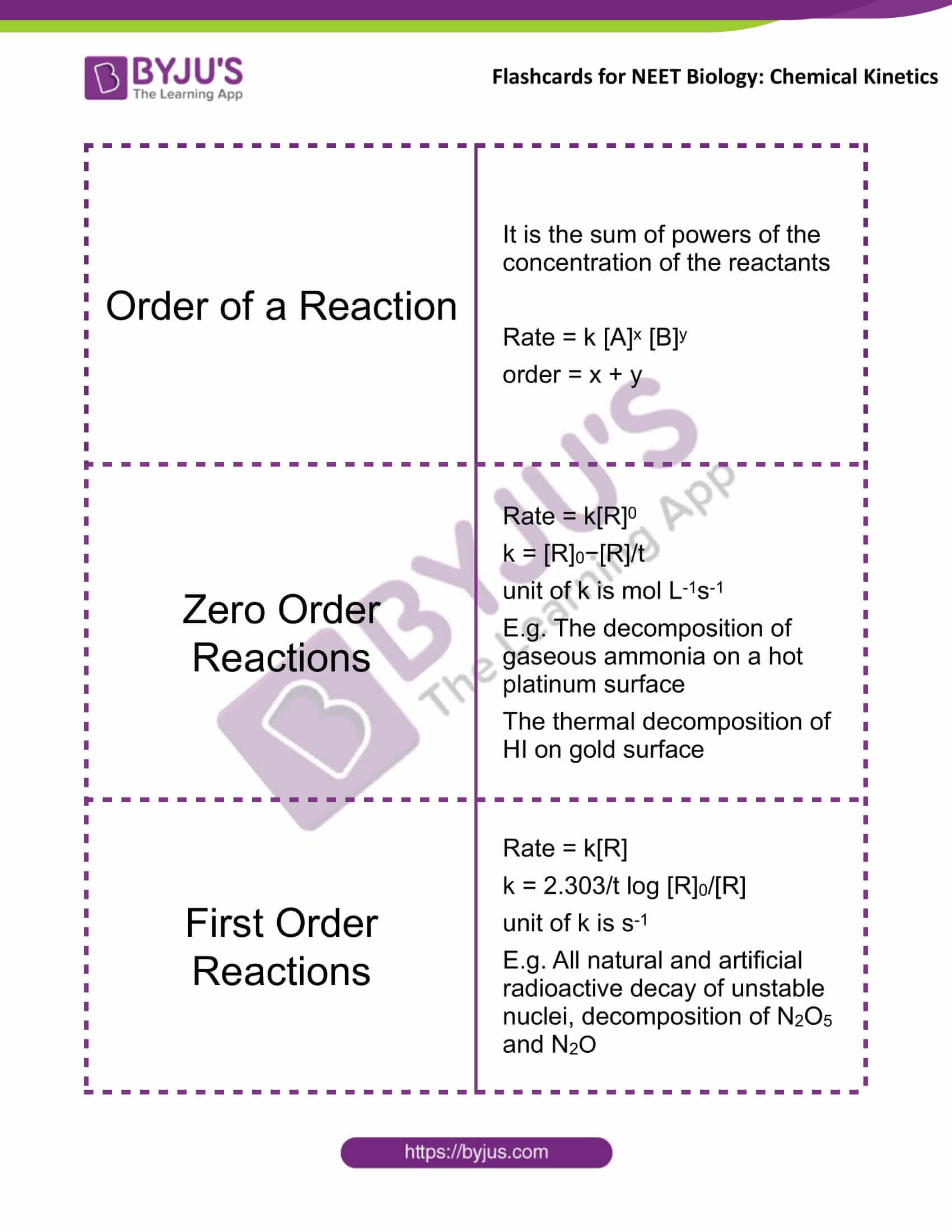
Order of a Reaction |
It is the sum of powers of the concentration of the reactants Rate = k [A]x [B]y order = x + y |
|
Zero-Order Reactions |
Rate = k[R]0 k = [R]0−[R]/t unit of k is mol L-1s-1 E.g. The decomposition of gaseous ammonia on a hot platinum surface The thermal decomposition of HI on gold surface |
|
First-Order Reactions |
Rate = k[R] k = 2.303/t log [R]0/[R] unit of k is s-1 E.g. All natural and artificial radioactive decay of unstable nuclei, decomposition of N2O5 and N2O |
|
Half-Life of a Reaction |
It is the time in which the concentration of a reactant becomes half of its initial concentration. It is denoted by t1/2. For zero order reaction t1/2 ∝ [R]0 or initial concentration t1/2 = [R]0/2k For first order reaction t1/2 is independent of [R]0 and is equal to 0.693/k |
|
Collision Frequency |
It is the number of collisions per second per unit volume Rate of reaction = ZAB e−Ea /RT ZAB – collision frequency of reactants e−Ea /RT – fraction of molecules with energies equal or more than Ea (activation energy) |
|
Arrhenius Equation |
It explains the temperature dependence of the rate of a reaction k = A e-Ea /RT A – Arrhenius factor or the frequency factor Ea – activation energy in joules/mole (J mol –1) |
|
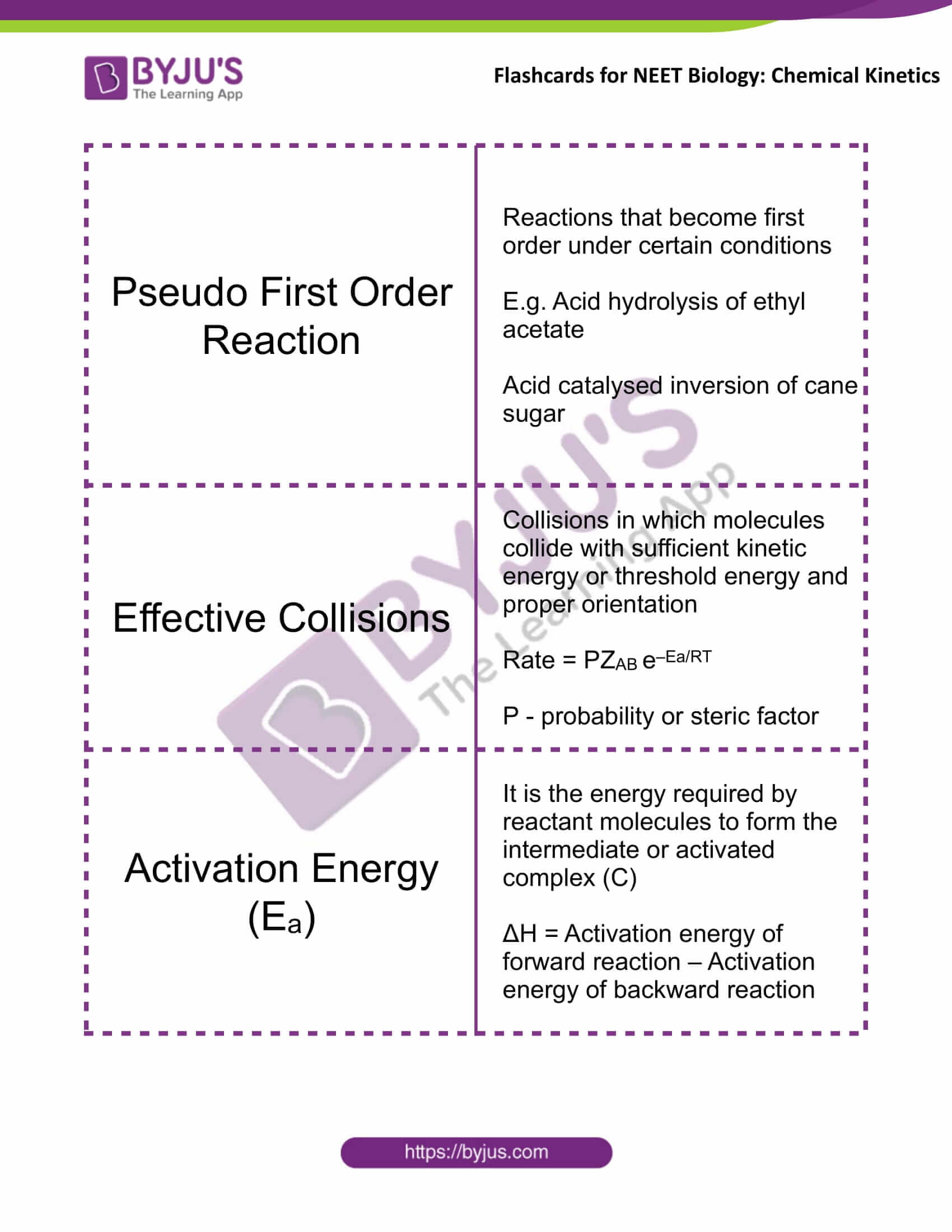
Pseudo First Order Reaction |
Reactions that become first order under certain conditions E.g. Acid hydrolysis of ethyl acetate Acid catalysed inversion of cane sugar |
|
Effective Collisions |
Collisions in which molecules collide with sufficient kinetic energy or threshold energy and proper orientation Rate = PZAB e–Ea/RT P – probability or steric factor |
|
Activation Energy (Ea) |
It is the energy required by reactant molecules to form the intermediate or activated complex (C) ΔH = Activation energy of forward reaction – Activation energy of backward reaction |
Get access to the full set of flashcards for NEET Chemistry, only at BYJU’S.
|
Also check: |

Comments