NEET 2023 is approaching, and it is time to do thorough revisions and give your best for the entrance exam. This comprehensive is based on the importance, weightage, and previous years’ trends for NEET 2023. Some of the topics covered in this Smart Mock Test are the Monomer of neoprene polymer, Fehling’s solution test, the iodoform test, and electron bonds.
Question 1: Which among the following species is pyramidal in shape?
- ClF3
- SF4
- NF3
- BCl3
Answer: c) NF3
Explanation: When the central atom in a molecule has three sigma bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. Here, NF3 has 3 sigma bonds and one lone pair.
Question 2: The volume strength of the 0.75 M H2O2 solution is:
- 8.4
- 0.26
- 3.0
- 7.5
Answer: a) 8.4
Explanation: 2H2O2 → 2H2O + O2
2 x 34 22.4
0.75M H2O2 = 0.75 x 34g of H2O2
Hence volume strength = (0.75 x 34 x 22400) / (68 x 1000) = 8.4
Question 3: Packing fraction of body-centred cubic structure is:
- π/6
- √3/8π
- √2/8π
- √3/6π
Answer: b) √3/8π
Question 4: The Monomer of neoprene polymer is:
- 2-Chloro-1,3-butadiene
- 1−Chloro−1,3−butadiene
- 2-Methyl-1,3-butadiene
- 3-Hydroxybutanoic acid
Answer: a) 2-Chloro-1,3-butadiene
Explanation: Neoprene is a rubber-like addition homopolymer having rubber-like characteristics.
Chloroprene (CH2=C(Cl)−CH=CH2) is the Monomer of neoprene.
Chloroprene is also known as 2-Chloro-1,3-butadiene.
Question 5: The number of 3-centre-2-electron bonds in B2H6 is:
- 3
- 4
- 2
- 6
Answer: c) 2
Explanation: When BH3 dimerizes, it forms the diborane B2H6, which has two bridging hydrogen atoms and four-terminal hydrogen atoms. The two electrons in a three-centre two-electron bond are shared by three atoms.
Banana bonds are another name for these bonds.
Question 6: Which one of the following alkali metals can directly react with N2 of air?
- Li
- Na
- K
- Rb
Answer: a) Li
Explanation: Li is an alkali metal that reacts directly with nitrogen. Because of its small size and great polarising power, it exhibits anomalous behaviour.
6Li + N2 → 2Li3N
Hence, option ‘a’ is correct.
Question 7: Which of the following gives both a positive iodoform test and Fehling’s solution test?
- Acetaldehyde
- Acetone
- Ethyl alcohol
- Formaldehyde
Answer: a) Acetaldehyde
Explanation: Acetaldehyde produces positive iodoform and Fehling’s solution tests.
In the presence of NaOH, the compound containing the CH3−C− group gives a positive result with the iodoform test, producing a pale yellow iodoform precipitate.
The presence of aldehyde, but not ketone, is identified in Fehling’s test by reducing a deep blue copper (II) solution to a red precipitate of insoluble copper oxide.
Because it contains both an s−∂ carbon atom and an aldehyde group, only acetaldehyde will pass both tests.
Question 8: Which of the following is most acidic in nature?
- HClO
- HClO2
- HClO3
- HClO4
Answer: d) HClO4
Explanation: Oxygen has a higher electronegative charge than chlorine. More electrons are pushed away from the O−H bond as the number of O atoms linked to Cl increases, making the O−H bond weaker. The acid strength is increased as a result of this. Thus, HClO4 is the most acidic in nature among the given list.
Question 9: The species which does not exist is:
- H2
- He2
- C2
- O2
Answer: b) He2
Explanation: The Bond Order (B.O.) of He2 is zero. Hence, it does not exist.
B.O. = 1/2 [Bond e– − Anti bond e–] = 1/2 [2 − 2] = 0.
Question 10: When copper is reacted with dilute HNO3, the gaseous product(s) obtained is/are:
- NO2 only
- N2O only
- NO only
- N2O and NO2
Answer: c) NO only
Explanation: Copper reacts with dilute HNO3 to yield copper nitrate, water and nitric oxide.
3Cu + dil. 8HNO3 → 3Cu(NO3)2 + 4H2O + 2NO
Question 11: Correct order of Van der Waals constant b of given gases is:
- CO2 > N2 > O2 > H2 > He
- N2 > CO2 > O2 > H2 > He
- CO2 > N2 > O2 > He > H2
- N2 > O2 > H2 > He > CO2
Answer: a) CO2 > N2 > O2 > H2 > He
Explanation: As we know, Van der Waals constant b ∝ Size. Individual gases have these qualities, which can also have positive values. The constants in an ideal gas tend to be zero, and PV = nRT is satisfied.
This compensates for intermolecular forces. There are two ‘a’ and ‘b’ Van der Waals constants. These two constants are connected to molecular volume and attractive forces in general.
The boiling points of molecules are often directly proportional to Van der Waal forces, while the distance between two atoms is inversely proportional.
Question 12: For the reaction, CaCO3 (s) → CaO (s) + CO2 (g) ; ∆H = 82.8 kJ at 25°C
∆U at 25°C is:
- 85.28 kJ
- 82.8 kJ
- 80.32 kJ
- 234 kJ
Answer: c) 80.32 kJ
Explanation: For the given reaction –
CaCO3 (s) → CaO (s) + CO2 (g)
Δng = nP − nR = 1− 0 = 1
ΔH = 82.8 kJ
T = 25℃ = (25 + 273)K = 298K
Now, as we know that,
ΔH = ΔU + ΔngRT
⇒ ΔU = ΔH − ΔngRT
⇒ ΔU = 82.8 − (1×8.314×10−3 × 298)
⇒ ΔU = 82.8 − 2.48 = 80.32kJ
Thus, the value of ΔU or ΔE at 25℃ is 80.32kJ.
Question 13: pH for 0.01 M NaOH solution is:
- 2
- 12
- 10
- 8
Answer: b) 12
Explanation: The pH is given by,
pH = −log[H+]
pH + pOH = 14
pOH = −log[OH−]
Given:
Conc. of NaOH = 0.01M
Conc. of NaOH ions = 0.01M = 10−2M
pOH = −log[OH−]
⇒ pOH = −log(10−2)
⇒ pOH = 2 log 10 = 2
pH + pOH = 14
∴ pH = 14 − 2 = 12
Thus, the pH of 0.01M NaOH solution is 12.
Question 14: Maximum number of electrons present in the p-subshell is:
- 2
- 6
- 10
- 5
Answer: b) 6
Explanation: An electron shell, also known as a principal energy level, is an orbit that electrons follow around the nucleus of an atom. Only a certain amount of electrons can fit into each shell: The first shell can hold two electrons, the second shell has eight (2 + 6) electrons, the third shell has 18 (2 + 6 + 10) electrons, and so forth. Each subshell has one or more atomic orbitals, and each shell has one or more subshells.
Each subshell is limited to a maximum of 4ℓ + 2 electrons, i.e.
Each s subshell can only hold 2 electrons.
Each p subshell can only hold 6 electrons.
Each d subshell can only hold a maximum of 10 electrons.
There are 14 electrons in each f subshell.
Each g subshell can only hold 18 electrons at a time.
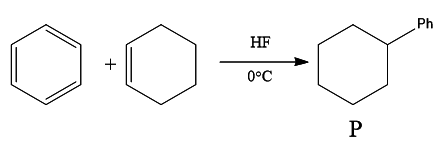
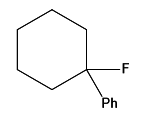
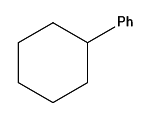
Question 15: In the given reaction, the product P is:
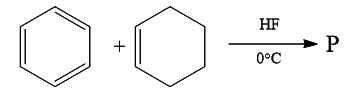
Answer: c) 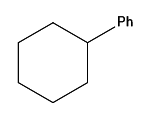
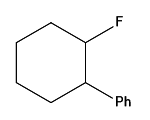
Explanation: It is not the same as the Friedel-Crafts reaction. An electrophile is formed when the acid produces a cyclohexyl secondary carbocation:
Question 16: The number of isomers for the complex species [Co(en)2Br2]+ is:
- 6
- 3
- 4
- 2
Answer: b) 3
Explanation: For the compound [Co(en)2Br2]+, the number of potential isomers will be (en = ethylenediamine) 3. The geometrical isomers cis and trans exist. The trans isomer is optically inactive, but the cis is available in two forms: d and l.
Question 17: Consider the following reaction sequence,
- CH3 − CH = CH − CHO
- CH3 − CH = CH − CH2 − OH
- CH3 − CH = CH − CH3
- CH3 − CH2 − CH2 − CH3
Answer: c) CH3 − CH = CH − CH3
Question 18: The correct order of basic strength is:
- Aniline > p-nitroaniline > p-toluidine
- p−nitroaniline > Aniline > p−toluidine
- p-toluidine > Aniline > p-nitroaniline
- p-toluidine > p-nitroaniline > Aniline
Answer: c) p-toluidine > Aniline > p-nitroaniline
Explanation: Because it contains the electron-donating group −CH3, p-toluidine has the strongest basic strength of the three compounds: aniline, p-nitroaniline, and p-toluidine. The inclusion of the (−NO2) group in p-nitroaniline, on the other hand, reduces its electron density across the N-atoms. As a result, it is the weakest of the three bases.
Recommended Video:

Related Links:
Comments