What is surface tension? It is a phenomenon which causes the surface of the liquid to behave like an elastic sheet. If the phenomenon occurs in between two types of liquids, it is called interface tension. By virtue of the property of surface tension, liquids always tend to acquire the least surface area possible.
A water drop is always in the shape of a sphere although a falling drop may adopt various shapes, because of various forces acting on it. Because of surface tension, the wall tension required for the formation of drops or bubbles is provided. The tendency to minimise surface area, causes the wall tension to be pulled inwards, directed at all sides, thereby leading to a spherical shape.
| Table of Contents |
Cohesive and Adhesive Forces
Cohesive forces are the forces of attraction between molecules of a similar type. For example, the forces of attraction between molecules of water in a glass. Adhesive forces, on the other hand, are forces of attraction between molecules of different types. For example, the force of attraction between water molecules in a glass and the glass molecules.
To know more differences between adhesion and cohesion, click on the link below:
Capillary Rise
We all know that plants absorb water from the soil to make food (photosynthesis). But have you ever wondered how this happens? For water to rise up, it has to work against gravity and yet it does happen. This is another phenomenon which occurs because of the surface tension of liquids.
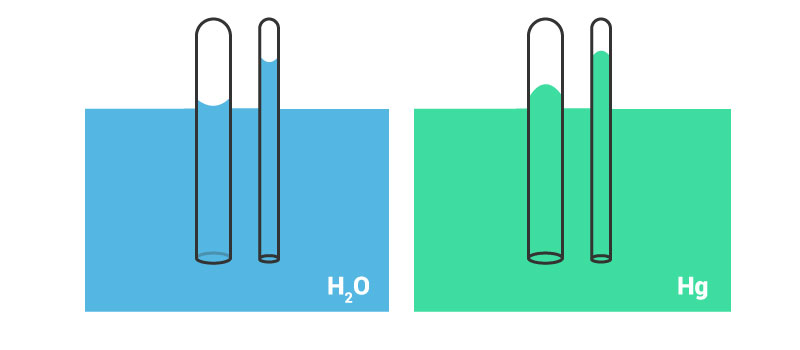
If water is placed in a beaker or a narrow measuring cylinder, you can see that the surface of the water meniscus isn’t straight. It forms a slight depression. Actually, due to adhesive forces between water and surface, the outer edge is pulled upwards (in case of water). An image illustrating this effect is shown below.

The film formed due to surface tension tends to hold the surface in place and due to this, the entire liquid is pulled upwards, when the edges are pulled upwards.
Say a very thin and long narrow tube is placed in a tub of water, adhesive forces will cause the water to rise a bit in this tube, won’t it? When the adhesive forces are greater than the cohesive forces between water molecules, the water tends to rise.
The height to which the water rises is given by the following relation.
h = 2σ / ρrg
where,
- h is the height of rising of liquid due to capillary action
- σ is the surface tension of the liquid
- ρ is the density of the liquid
- g is the acceleration due to gravity
- r is the radius of the tube
What do you think will happen if the cohesive forces are greater than the adhesive forces? Maybe the image given below will give you a hint.

thanks!!!!!!!!!!!!!!!!!!!!!!!!!!