Potassium Iodide formula is one of the easiest formulas to remember. However, before learning the formula let us quickly read about Potassium iodide. It is an ionic compound which is produced industrially by treating potassium hydroxide (KOH) with iodine. This inorganic compound is a white crystalline salt which has wide ranges of uses in many fields like medicine, commercial and industrial applications. The white solid usually turns yellow in colour when it is exposed to air where oxidation occurs.
Potassium Iodide Chemical Formula
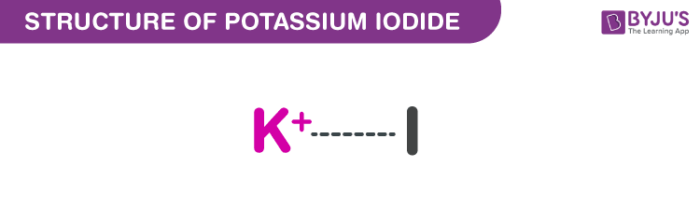
Potassium iodide is made of ions such as K+I and behaves like an ionic salt. As they are ionically bonded together, potassium has an oxidation number of +1 and iodide is -1. Since there is a tendency to gain or lose electrons to achieve a stable state we get the formula KI.
| Formula | KI |
| Molar Mass | 166.0028 g·mol−1 |
| Density | 3.12 g/cm³ |
| Melting Point | 681 °C |
| Boiling Point | 1,330 °C |
Potassium Iodide Structural Formula
Keep visiting BYJU’S to access pages of different formulas of important chemical compounds.
Comments