Potassium Permanganate Formula is given here along with its structure. Potassium permanganate is an inorganic compound and is also known as hypermangan or Condy’s crystals. It is water-soluble and a strong oxidizing agent. Learn more about Potassium permanganate here along with its physical and chemical properties, uses, and structure, and other details.
Potassium Permanganate Chemical Formula
Potassium permanganate is an ionic compound consisting of potassium ion (acting as a cation) and permanganate (acting as an anion). It has a molar mass of 158.034 g/mol and its chemical formula is given as-
|
Potassium Permanganate Chemical Formula: KMnO4 |
Potassium Permanganate Structural Formula
Potassium Permanganate consists of 1 potassium atom, 1 Manganese atom, and 4 oxygen atoms. One manganese atom is attached to the four oxygen atoms in the permanganate anion. The structure of potassium permanganate is-
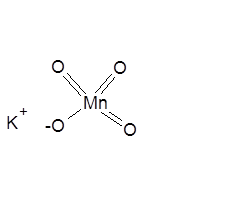
Potassium Permanganate is a strong oxidizing agent and is used extensively in treating ulcers, sores, fungal infections, dermatitis, and other skin conditions due to its antiseptic and non-toxic (when diluted) properties. It is prepared by reacting manganese dioxide with potassium hydroxide and then by electrolytic oxidation. Check out the Preparation of Potassium Dichromate and Potassium Permanganate here to know the complete reaction process.
Stay tuned with BYJU’S to get more formulas of different chemical compounds and to get other preparation materials for the exams.
Thanks you for the explanation 😊