Selenic acid is a strong dibasic acid with the formula H2SeO4. It can be manufactured by oxidising selenium compounds in lower oxidation states. One of the methods includes oxidising selenium dioxide with hydrogen peroxide.
\(\begin{array}{l}SeO_2+H_2O_2\rightarrow H_2SeO_4\end{array} \)
In this short piece of article, we will be discussing more the selenic acid formula, its chemical structure properties and uses.
Selenic Acid Properties
| Properties of Selenic Acid | |
| Name | Selenic Acid |
| Other Names | Selenic (VI) Acid |
| Appearance | White or Colourless crystals |
| Chemical Formula | H2SeO4 |
| Melting Point | 58 °C |
| Boiling Point | 260 °C |
| Density | 2.95 g/cm3 |
| Molar Mass | 144.9734 g/mol |
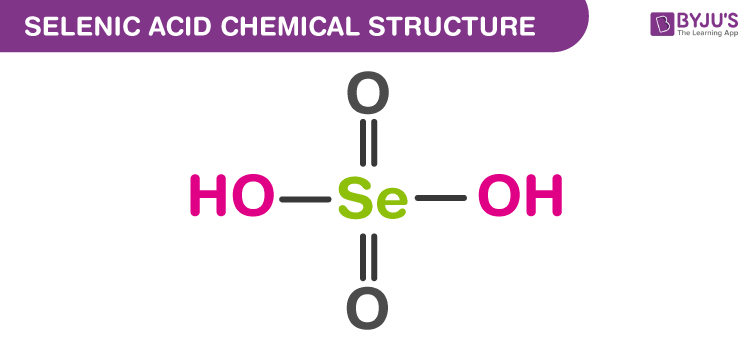
Selenic Acid Structure
Selenic Acid Uses
- Used as a specialised oxidising agent
- Used in metal refining, rust removal, fertilizer manufacture and photography
- Used in metal, drain and toilet cleaners
To learn more about such chemistry topics register to BYJU’S now!
Comments