Sodium Bromide is an inorganic compound with the chemical formula NaBr. It is a white crystalline solid and its appearance closely resembles the appearance of table salt. It is produced by treating sodium hydroxide with hydrogen bromide. In this short piece of article, let us learn more about the sodium bromide formula, its chemical structure, sodium bromide properties and its uses.
Sodium Bromide Properties
| Properties of Sodium Bromide | |
| Name | Sodium Bromide |
| Appearance | White crystalline solid |
| Molecular Formula | NaBr |
| Melting Point | 747 °C (anhydrous)
36 °C (dihydrate) |
| Boiling Point | 1,396 °C |
| Density | 3.21 g/cm³ (anhydrous)
2.18 g/cm3 (dihydrate) |
| Molar Mass | 102.894 g/mol |
| Solubility in Water | Soluble in Water |
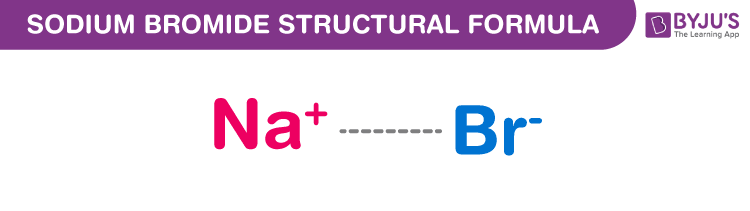
Sodium Bromide Structure
Sodium Bromide Uses
- Used as a disinfectant for hot tubs and swimming pools
- Used to prepare dense fluids that are used in oil wells.
- The photosensitive salt, silver bromide, is prepared using sodium bromide
- Used as a catalyst in TEMPO-mediated oxidation reactions
To learn more about such chemistry topics register to BYJU’S now!
Comments