Sodium Chlorate is an inorganic chemical compound with a formula NaClO3. It a white crystalline powder that is hygroscopic in nature. Sodium chlorate can be produced by the electrolysis of hot sodium chloride solution. The other name of sodium chlorate is sodium chlorate (V). In this short piece of article, learn more about the sodium chlorate formula, its properties and chemical structure along with its uses.
Sodium Chlorate Properties
| Properties of Sodium Chlorate | |
| Name | Sodium Chlorate |
| Appearance and odour | Pale Yellow to white crystalline solid
Odour – Odourless |
| Chemical Formula | NaClO3 |
| Melting Point | 248 °C |
| Boiling Point | 300 °C |
| Density | 2.5 g/cm³ |
| Molar Mass | 106.44 g/mol |
| Solubility in Water | Soluble |
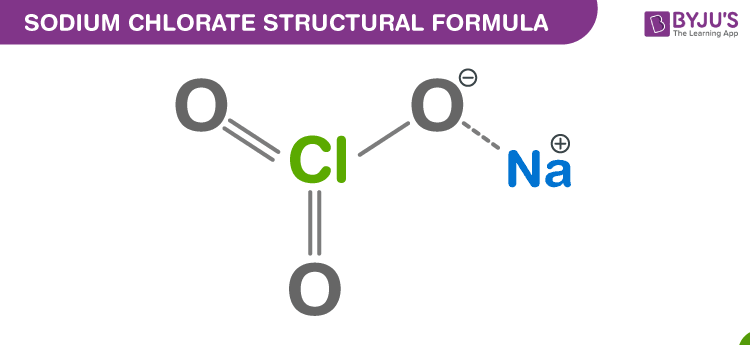
Sodium Chlorate Chemical Structure
Sodium Chlorate Uses
- Used in the pulp and paper industry
- Used to produce chlorine dioxide
To learn more such chemistry formula of various chemical compounds, Visit BYJU’S.
Comments