Sodium Fluoride formula, also known as Florocid formula or Pediaflor formula is explained in this article. This inorganic ionic molecule dissolves in water to give Na+ and F− ions. It consists of one atom of sodium and one atom of fluorine. It can be prepared by neutralizing hydrofluoric acid or hexafluorosilicic acid with sodium hydroxide. Sometimes alcohols are also used to precipitate the NaF. The chemical or molecular formula of Sodium Fluoride is NaF.
Florocid is obtained either as a colourless crystalline solid or the solid dissolved in a liquid, or white powder. It is odourless and has a salty taste. It is soluble in water and insoluble in alcohol. This non-combustible compound dissolves in water and is corrosive to aluminium. It is widely used as an insecticide, wood preservative, glass manufacturing, cleaning compounds, etc.
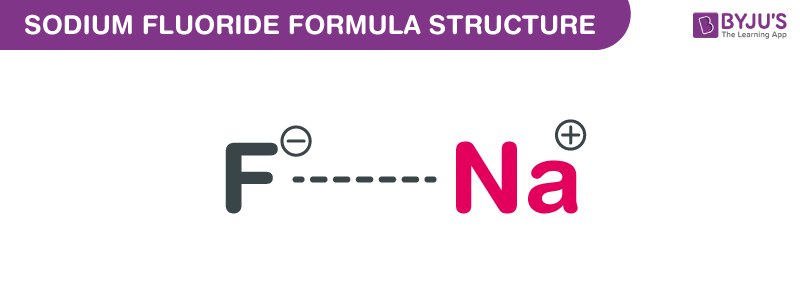
Sodium Fluoride Formula Structure
Properties Of Sodium Fluoride Formula
| Chemical formula | NaF |
| Molecular weight | 41.988173 g/mol |
| Density | 2.558 g/cm3 |
| Boiling point | 1,704 °C |
| Melting point | 993 °C |
The mineral form of Sodium Fluoride viz Villiaumite is moderately rare. It is known from a type of rocks called Plutonic Nepheline Syenite Rocks.
To learn more about Sodium Fluoride formula from the expert faculties at BYJU’S, register now!
Comments