Stearic Acid is a waxy white solid with a mild odour and by nature floats on top of the water.
plant and animal fats are the major sources of Stearic Acid and mainly shea butter and cocoa butter are the major sources. Stearic Acid is used in softening plastics, hardening soaps and in the production of candles cosmetics, and plastics.
Properties Of Steric Acid
| Chemical formula | CH3(CH2)16COOH or C18H36O2 |
| Molecular weight | 284.484 g/mol |
| Chemical names | Octadecanoic acid, 57-11-4
Stearophanic acid, n-Octadecanoic acid |
| Density | 0.9408 g/cm3 (20 °C)[2]
0.847 g/cm3 (70 °C) |
| Boiling point | 361 °C (682 °F; 634 K)
decomposes |
| Crystal structure | Monoclinc |
Stearic Acid Structural Formula
Stearic Acid features 18 carbon backbone straight-chain structure as shown in the figure below.
![]()
Pictorial representation
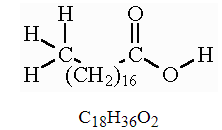
Structural Representation
Also, Read ⇒ Structure & Uses of Stearic Acid
For more interesting information on chemical components, visit BYJU’S!!
Comments