Strontium chloride, or also known as strontium dichloride, is an inorganic salt with a chemical formula SrCl2 is widely used in dental products and fireworks. From the formula, we get to know that it is made of a strontium cation and two chloride anions. In this short piece of article, learn more about the strontium chloride formula, its chemical structure along with its properties and uses.
Strontium Chloride Properties
| Properties of Strontium Chloride | |
| Name | Strontium Chloride |
| Also known as | Strontium dichloride, frozone |
| Appearance | White Crystalline Solid |
| Chemical Formula | SrCl2 |
| Melting Point | 874 °C (anhydrous)
61 °C (hexahydrate) |
| Boiling Point | 1,250 °C (anhydrous) |
| Density | 3.05 g/cm³ (anhydrous, monoclinic form)
2.672 g/cm3 (dihydrate) 1.930 g/cm3 (hexahydrate) |
| Molar Mass | 158.53 g/mol (anhydrous monoclinic form)
266.62 g/mol (hexahydrate) |
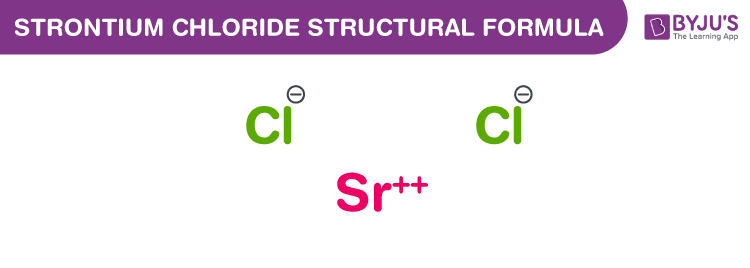
Strontium Chloride Chemical Structure
Strontium Chloride Uses
- Used as a corrosion inhibitor for aluminium
- Used as a red colouring agent in pyrotechnics
- Useful in reducing tooth sensitivity
- Used in the storage of ammonia
To learn more about such chemistry topics register to BYJU’S now!
Comments