Even if it’s an essential part of the TN Board Exams, students often forget or overlook the practicals, regarding them as unimportant. However, practicals have a massive significance in the learning process as they help to engage students. It helps them to develop essential skills and develop a broad understanding of the Scientific concepts. Tamil Nadu Class 10 Science Practicals are also an important part of the board exams and students need to be proficient in it if they wish to score high marks in the exams.
In this article, we have provided a list of experiments that have to be performed by the students for Tamil Nadu Board Class 10 Science Practical for this academic year. These experiments have been collected based on the Samacheer Kalvi Class 10 books. From here, students can find the details that are required by them to complete the practicals, which is based on the TN Board Class 10 syllabus.
Download Tamil Nadu Board Class 10 Science Practical Free PDF
Here we have given the table of contents and list of experiments.
TN Board Class 10 Science Practical – Table of Contents
| S. No. | Name of the Experiment | Time | |
| 1 | Physics | Determination of the weight of an object using the principle of moments | 40 min |
| 2 | Determination of focal length of a convex lens | 40 min | |
| 3 | Determination of resistivity | 40 min | |
| 4 | Chemistry | Identification or the dissolution of the given salt whether it is exothermic or endothermic | 40 min |
| 5 | Testing the solubility of the salt | 40 min | |
| 6 | Testing the water of hydration of salt | 40 min | |
| 7 | Test the given sample for the presence of acid or base | 40 min | |
| 8 | Bio-Botany | Photosynthesis -Test tube and Funnel Experiment
(Demonstration) |
40 min |
| 9 | Parts of a Flower | 40 min | |
| 10 | To Study the Law of Dominance | 40 min | |
| 11 | Observation of Transverse Section of Dicot stem and Dicot Root | 40 min | |
| 12 | Bio-Zoology | Observation of Models – Human Heart and Human Brain | 40 min |
| 13 | Identification of Blood Cells | 40 min | |
| 14 | Identification of Endocrine Glands | 40 min | |
Tamil Nadu Board Class 10 Practicals Science – List of Experiments
Students should try to score well in the practical exams so that the added marks would help them to improve the overall score. Meanwhile, the experiments are explained in an easy format with instructions for the students to follow for accurate execution.
Description of the experiment in the TN Class 10 Science Practical book follows the given format:
- Aim of the Experiments
- Apparatus or Materials Required
- Formula or Principle behind the Experiment
- Procedure
- Observations
- Result
Check the List of Class 10 Science Experiment from below:
List of Experiments from Physics
1. DETERMINATION OF THE WEIGHT OF AN OBJECT USING THE PRINCIPLE OF MOMENTS
Aim: To determine the weight of an object using the principle of moments
Apparatus required: A metre scale, a knife-edge, slotted weights, thread
Procedure:
i. A metre scale is supported at its centre of gravity by a knife-edge or suspended by
using a thread tied to its centre so that the scale is in the horizontal position. Ensure that
the scale is in an equilibrium position.
ii. A known weight W2 and an unknown weight W1 are suspended from either side of the
scale using the weight hangers.
iii. Fix the position of one weight hanger and adjust the position of the second weight hanger
such that the scale is in equilibrium.
iv. Measure the distance d1 and d2 of the two weight hangers from the centre of the scale
accurately.
v. The experiment is repeated for different positions of the unknown weight. Measure the
distances. The readings are tabulated as follows:
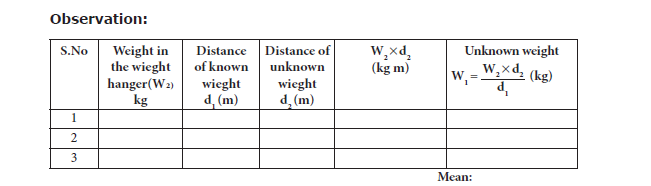

Result: Using the principle of moments, the weight of the unknown body W1 = ……. Kg Wt.
2. DETERMINATION OF FOCAL LENGTH OF A CONVEX LENS
Aim: To determine the focal length of a convex lens by using
1. Distant object method
2. UV method
Apparatus required: A convex lens, stand, wire gauze object, screen and measuring scale.

1. Distant Object Method: Fix the given convex lens vertically on the stand and place it on the table near an open window of the laboratory. Locate a distant object (tree or building) through the open window. Place the screen behind the convex lens. Adjust the position of the convex lens and the screen so as to get a sharp, inverted and diminished image. Measure the distance between the screen and the convex lens with the help of the measuring scale. This distance is equal to the approximate focal length of the convex lens (f)
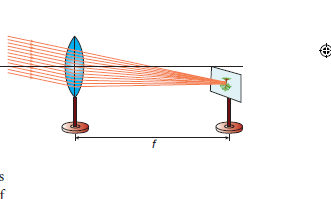
2. UV – Method: Fix the given convex lens vertically on the stand and place it on the table. Place the wire gauze object on the left side of the convex lens (say at a distance greater than
2f). Measure the distance between the object and the lens (u). Place the screen on the right
side of the convex lens and adjust its position to get a sharp, inverted and diminished
image. Measure the distance between the screen and the lens (v). Repeat the same procedure, by changing the distance of the object (u) and tabulate your observations.

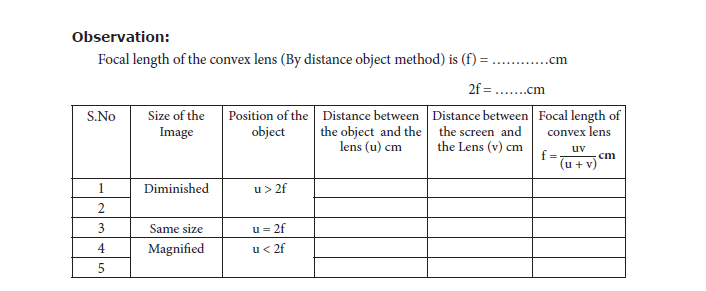
Result: The focal length of the given convex lens
1. By distance object method f = ………cm
2. By ‘uv’ method f = … .…..cm
3. DETERMINATION OF RESISTIVITY
Objective: To determine the resistivity of the material of the given coil of wire.
Equipment required: A coil of wire, screw gauge, a metre scale, battery, key, ammeter, voltmeter, rheostat and connecting wires.
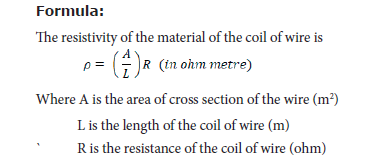
Circuit Diagram:
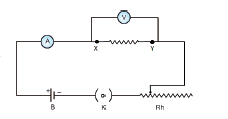
Procedure:
• Connect the battery, ammeter, given wire, rheostat and key in series, as shown in the circuit diagram.
• Connect the voltmeter in parallel to the unknown resistor.
• Close the key and hence the circuit is closed.
• Adjust the rheostat such that the ammeter reads a current of 0.5 ampere.
• Note down the potential difference across the resistor as shown by the voltmeter.
• Adjust the rheostat and change the current in steps of 0.5A (0.5A, 1.0A, 1.5A, etc.).
• For each current, note down the corresponding potential difference as shown by the voltmeter.
• Tabulate the observations.
• Measure the diameter of the wire using a screw gauge.
• Measure the length of the coil using a metre scale
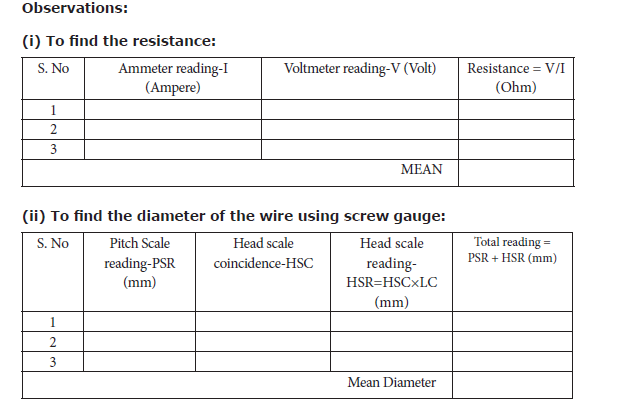
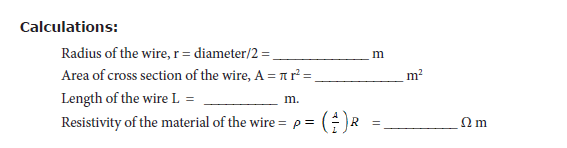
Result: The resistivity of the material of the wire = __________ Ω m
List of Experiments from Chemistry
4. IDENTIFY THE DISSOLUTION OF THE GIVEN SALT WHETHER IT IS EXOTHERMIC OR ENDOTHERMIC.
Aim: To test the dissolution of given salt is exothermic or endothermic
Principle:
If the reaction or process liberates the heat, then it is called exothermic.
If the reaction or process absorbs the heat, then it is called endothermic
Apparatus required: Two beakers, Thermometer, stirrer, weighed amount of two samples.
Procedure:
Take 50ml of water in two beakers and label them as A and B. Note the temperature of
the water from the beaker A and B. Then, add 5g of sample A into the beaker A and stir well until it dissolves completely. Record final temperature of the solution. Now, repeat the same for the sample B. Record the observation.
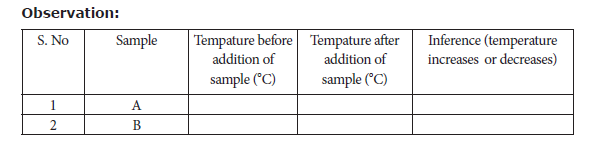
Result: From the inferences made
The dissolution of sample A is ____________________(Exothermic or endothermic)
The dissolution of sample B is ____________________(Exothermic or endothermic)
Note: Sodium hydroxide, ammonium nitrate, glucose, calcium oxide etc. may be given as the sample.
5. TESTING THE SOLUBILITY OF THE SALT
Aim: To test the solubility of the given salt based on the saturation and unsaturation
of the solution at a given temperature.
Principle:
A solution in which no more solute can be dissolved in the solvent at a given temperature is called a saturated solution. If the solvent can dissolve more solute than what is present, the solution is called the unsaturated solution.
Materials Required: A 250 ml beaker, a stirrer, a sufficient quantity of distilled water, 100 ml measuring jar, table salt in three packets weighing as 25g, 11g, and 1g.
Procedure:
In a 250ml beaker, pour 100 ml water using measuring jar. To this water add table salt (25 g) from the first packet. Stir the content very well. Add the next packet containing 11 g salt followed by constant stirring. Now add the third packet containing 1 g salt. Record your observations.
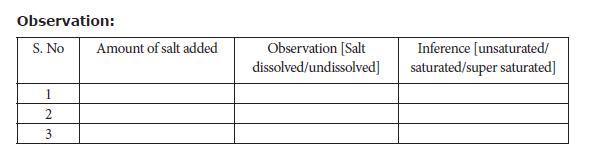
Result: From the above observation, it is inferred that the amount of salt required for saturation is _______ g
6. TESTING THE WATER OF HYDRATION OF SALT
Aim: To check whether the given sample of salt possesses ‘Water of Hydration’ or
not. To verify the presence of water molecules in the given hydrated salt.
Principle:
The water of crystallization or water of hydration is the phenomenon shown by certain salts in which water molecules are present inside the crystals and are responsible for their colour and geometry.
e.g. Crystalline copper sulphate CuSO4.5H2O
Materials Required: A pinch of crystalline copper sulphate in a test tube, tongs, spirit lamp.
Procedure: A pinch of crystalline copper sulphate taken in a test tube and heated for some time. Water droplets are seen on the inner walls of the test tube. This shows that the given salt
contains water of crystallization. If the above observation is not noticed for the given salt, the water of hydration is absent.

Result: In the given sample of salt, Water of crystallization/hydration is
A) Present
B) Absent
7. TEST THE GIVEN SAMPLE FOR THE PRESENCE OF ACID OR BASE
Aim: To identify the presence of an acid or a base in a given sample.
Materials Required: Test tubes, test tube stand, glass rod, phenolphthalein, methyl orange, sodium carbonate salt and the given sample.
Principle:
In an acid medium, In a base medium,
(a) Phenolphthalein is colourless (a) Phenolphthalein is pink in colour
(b) Methyl orange is pink in colour (b) Methyl orange is yellow in colour
(c) Sodium carbonate gives brisk effervescence (c) Sodium carbonate does not give brisk
effervescence
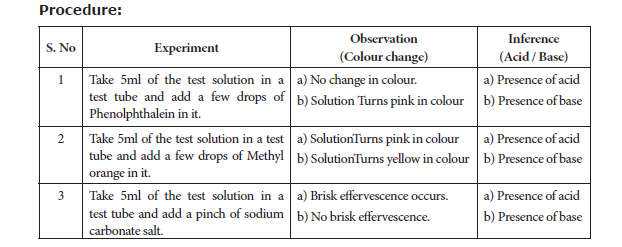
Result: The given test solution contains _____________ (acid / base).
List of Experiments from Bio-Botany
8. PHOTOSYNTHESIS-TEST TUBE AND FUNNEL EXPERIMENT (DEMONSTRATION)
Aim: To prove that oxygen is evolved during photosynthesis.
Materials required: Test tube, funnel, beaker, pond water and Hydrilla plant.
Procedure:
1. Take a few twigs of Hydrilla plant in a beaker containing pond water.
2. Place an inverted funnel over the plant.
3. Invert a test tube filled with water over the stem of the funnel.
4. Keep the apparatus in the sunlight for a few hours.
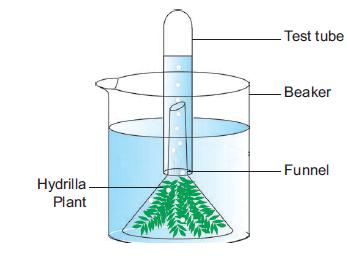
Observation: After one hour, it is noted that water gets displaced down from the test tube.
Inference: During photosynthesis, oxygen is evolved as a by-product. Gas bubbles liberated from the Hydrilla plant reach the top of the test tube and it displaces the water downwards. Take the test tube and keep the burning stick near the mouth of the test tube. The increased flame will appear. Hence, it is proved that oxygen is evolved during photosynthesis.
9. PARTS OF A FLOWER
Aim: To dissect and display the parts of the given flower and observe the Calyx, Corolla, Androecium and Gynoecium. Draw labelled sketches.
Materials Required: Flower, needle and paper
Procedure: With the help of the needle dissect the different whorls of the flower.
Floral Parts:
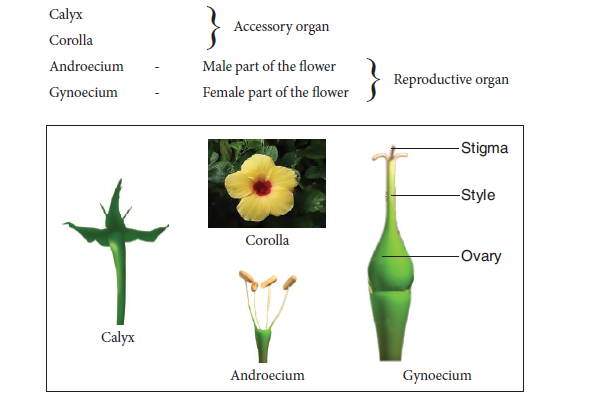
Observation: Draw and label the parts of the flower.
10. TO STUDY THE LAW OF DOMINANCE
Aim: To study the law of dominance by using model/picture / photograph. To find out the genotypic ratio and phenotypic ratio in pea plant using checkerboard
Procedure:
Depict parental generation and the gametes using colour chalk pieces
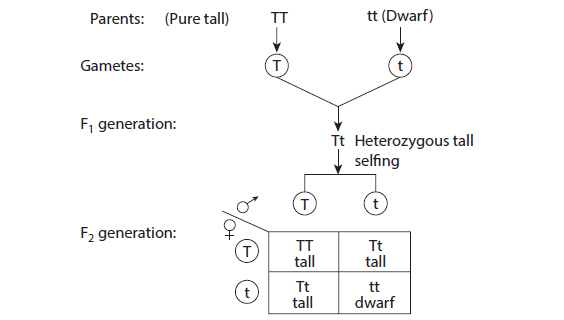
Result:
Phenotypic ratio 3:1
Genotypic ratio 1:2:1
Inheritance of only one pair of contrasting character is called monohybrid cross.
A cross between two forms of a single trait like a cross between tall and dwarf pea
plant.
11. OBSERVATION OF TRANSVERSE SECTION OF DICOT STEM AND DICOT ROOT
Aim: To observe transverse section (T.S) of Dicot Stem and Dicot Root from permanent slides.
Observation: A.The given slide is identified as T.S of Dicot Stem
T.S of Dicot Stem
(i) Vascular bundles are arranged in a ring.
(ii) Conjoint, collateral, endarch and open vascular bundle.
(iii) Ground tissued differentiated into cortex, endodermis, pericycle and pith.
(iv) 3 to 6 layer of collenchymas tissues present in hypodermis.

B.The given slide is identified as T.S of Dicot Root
T.S of Dicot Root
(i) Radial vascular bundle.
(ii) 2 to 4 xylem present
(iii) Cambium present
(iv) Cortex is made up of parenchymatous cells
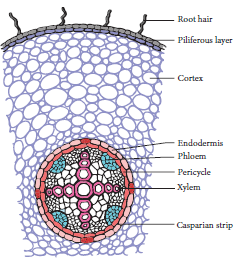
List of Experiments from Bio-Zoology
12. OBSERVATION OF MODELS-HUMAN HEART AND HUMAN BRAIN
Identification of longitudinal section (L.S) of the human heart.
Aim: To observe and draw a labelled sketch of L.S of the human heart and write the structure.
Materials Required: Model showing the L.S of the human heart
Observation: The given model is identified as L.S. of the human heart
1. The human heart has four chambers. It is made up of two auricles and two ventricles
2. The chambers are separated by interauricular and interventricular septum. It prevents the mixing of oxygenated and deoxygenated blood
3. Tricuspid valve – It is located between the right auricle and the right ventricle
4. Bicuspid valve – It is located between the left auricle and the left ventricle
5. The heart is covered by a protective double-walled membrane called pericardium
6. The heart pumps blood to all parts of the body

Identification of L.S of the human brain.
Aim: To observe and draw a labelled sketch of L.S of the human brain and indicate the different regions of the brain.
Materials Required: Model showing the L.S of the human brain
Identification: The given model is identified as L.S. of the human brain
1. The brain is enclosed in the cranial cavity
2. It is the controlling centre of all the body activities
3. It is covered by three connective tissue membrane or meninges: Duramater
Arachnoid membrane and Piamater
4. The human brain is divided into three parts namely forebrain, midbrain and hindbrain

13. IDENTIFICATION OF BLOOD CELLS
Aim: Identification of blood cells (Red blood cells and white blood cells). To draw a neat labelled diagram and write a note on the blood cells identified.
Materials Required: Permanent prepared slides of blood cells.
Identification: The given slide is identified as red blood cells
1. They are biconcave and disc-shaped
2. They are also known as erythrocytes
3. Mature mammalian RBCs do not have a nucleus
4. Haemoglobin is a respiratory pigment which gives red colour
5. It transports oxygen from lungs to tissues and carbon- dioxide from tissues to lungs

The given slide is identified as White blood cells
1. WBC’s are colourless and they have a nucleus
2. They are also known as Leucocytes
3. They show amoeboid movements
4. They fight against germs and other foreign bodies and thus protect the body from microbial infections and diseases
5. There are five different types of WBC named as Neutrophils, Eosinophils, Basophils, Lymphocytes and Monocytes

14. IDENTIFICATION OF ENDOCRINE GLANDS
Aim: To identify the endocrine gland, its location, a hormone secreted and functions – Thyroid gland and Pancreas
Materials Required:
1. Endocrine glands – (a) Thyroid gland (b) Pancreas – Islets of Langerhans
2. Anyone endocrine gland should be flag labelled. For the purpose of flag labelling a model / a chart/photograph showing all endocrine glands should be used. (Mark the endocrine glands mentioned for the practical)
Identification: Identify the flagged endocrine gland, write its location, the hormones secreted and its functions.

(a) Thyroid gland
Identification: The flag labelled endocrine gland is identified as Thyroid gland
Location: Thyroid gland is a bilobed gland located in the neck region on either side of the
Trachea.
Hormones secreted: Triiodothyronine (T3) and Thyroxine (T4)
Functions of Hormones:
1. Thyroid hormones increase the basal metabolic rate (BMR).
2. It increases body temperature
3. It regulates metabolism
4. It is required for normal growth and development
5. It is also known as personality hormone
6. Deficiency of thyroxine results in simple goitre, myxoedema (in adults) and cretinism (in children)
7. Excess secretion causes Grave’s diseases
(b) Pancreas – Islets of Langerhans
Identification: The flag labelled endocrine gland is identified as Islets of Langerhans in the pancreas.
Location: Islets of Langerhans are seen embedded in the pancreas which is located in the abdominal region.
Hormones secreted:
1. α cells secrete glucagon
2. β cells secrete insulin
Functions of Hormones:
1. Insulin converts glucose into glycogen and stores it in the liver and muscles.
2. Glucagon converts glycogen into glucose.
3. Insulin and glucagon maintain the blood sugar level (80 – 120 mg/dl) by their antagonistic
function.
4. Decrease in insulin secretion causes diabetes mellitus.
Stay tuned with Byju’s to get the latest notification on TN Board along with class 9 syllabus, sample papers, marking schemes and more.
Comments