When zinc oxide reacts with acetic acid zinc acetate is formed. Zinc Acetate commonly occurs as dihydrate and is in white crystalline in nature. It is used as a food additive and is also used to treat zinc deficiencies in humans. Zinc acetate is a white colour solid of all the forms.
Properties Of Zinc Acetate Structural Formula
| Chemical formula | C4H6O4Zn |
| Molecular weight | 183.468 g/mol (anhydrous)
219.50 g/mol (dihydrate) |
| Density | 1.735 g/cm3 (dihydrate) |
| Chemical names | Zinc diacetate, zinc salt, and Acetic acid, |
| Boiling point | decomposes |
| Melting point | Decomposes at 237 °C |
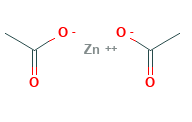
Zinc Acetate Structural Formula
The structural formula for zinc acetate is as shown in the figure below. It features tetrahedral molecular shape with octahedral geometry. The zinc is coordinated to 4 oxygen atoms to give an ionically balanced zinc acetate tetrahedral environment.
Stay tuned with BYJU’S for more scientific information!!
Comments