Zinc Bromide is a hygroscopic compound that is easily soluble in water. The zinc bromide forms di-hydrate as ZnBr2 · 2H2O. It is used in organic chemistry as a Lewis acid and also an electrolyte in the zinc bromide battery. It also used as a transparent film shield against radiations. Let us know more about the chemical composition and various other details of Zinc Bromide.
| Chemical Formula | Br2Zn |
| Molecular Weight | 225.188 g/mol |
| Chemical Name | Zinc dibromide, dibromozinc and Zinc(II) bromide |
| Melting Point | 394 °C |
| Solubility in water | 697 °C |
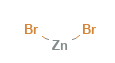
Zinc Bromide Structural Formula
Zinc bromide is a non-flammable chemical component. It has zinc with +2 charge and bromide ion of -1 charge. The structural formula of zinc bromide is as shown below in the diagram.
For more such interesting information on any other chemical compound, visit BYJU’S!
Comments