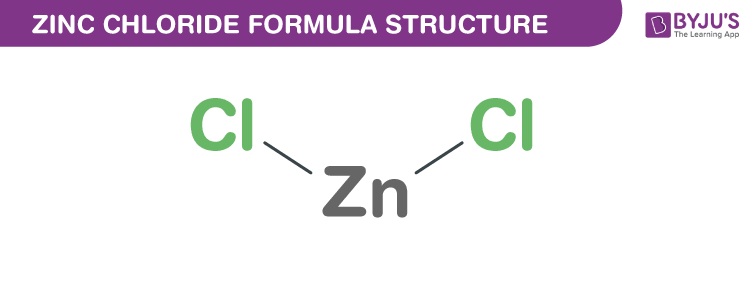
Zinc Chloride, commonly known as the butter of zinc, with a molecular formula ZnCl2, is a colourless or white crystal that is highly soluble in water. The zinc chloride molecule is a binary salt formed by the zinc cation Zn+2 and chloride anion Cl– and can be found as anhydrous or tetrahydrate form. In this short piece of article, let us learn more about the zinc chloride formula, its chemical structure, properties and uses.
Properties Of Zinc Chloride
| Zinc Chloride Properties | |
| Name | Zinc Chloride |
| Also Known as | Zinc Butter, Zinc dichloride |
| Appearance | Colourless or white crystals |
| Molecular Formula | ZnCl2 |
| Melting Point of Zinc Chloride | 275 °C |
| Boiling Point of Zinc Chloride | 756 °C |
| Density | 2.91 g/cm³ |
| Molar Mass | 136.286 g/mol |
| Solubility in Water | Soluble in water |
Zinc Chloride Chemical Structure
Zinc Chloride Uses
- Used in textile and paper industries
- Used as a disinfectant to prepare the Burnett’s Disinfecting Fluid
- Used as a catalyst in organic synthesis
To learn more about such chemistry topics register to BYJU’S now!
Comments